After the COVID-19 pandemic, the analysis of accumulated data could provide new insights to overcome further challenges for the healthcare system. The present study aimed to characterize the seropositivity levels in the context of COVID-19 morbidity in 2020–2022 and diagnostic, screening, and vaccination programs implementation. For this purpose, retrospective analysis of anti-SARS-CoV-2 IgG levels in 41,295 serum samples harvested from the northwestern Russian population in 2020–2024 was performed. We revealed that seroprevalence gradually increased until the autumn of 2021, when vaccination became mandatory for certain groups and restrictions were placed on unvaccinated persons. In the following winter, the seroprevalence growth was accompanied by a massive Omicron spread. The proportion of seropositive subjects in the Northwestern Russian population reached 80% seropositivity in 2022. Until the beginning of mass vaccination, the identified IgG levels in patients who reported suffering a non-COVID upper respiratory tract infection were significantly lower than in COVID-positive subjects. That suggests that the available diagnostic tests for SARS-CoV-2 provided patients and healthcare specialists with reliable information on the etiology of respiratory infections.
Keywords: COVID-19, SARS-CoV-2, seroprevalence, surveillance, vaccination
Almost immediately after the first COVID-19 case registration in Russia, on March 19, 2020, the Ministry of Health issued the order [1] that established the anti-pandemic measures, including a time limit for biological sample testing of 48 hours. Eventually, the Chief State Sanitary Doctor of Russia [2] mandated involving all government and private laboratories in COVID-19 diagnosis, and prescribed obligatory PCR testing for all citizens returning to Russia from overseas with respiratory tract infection (RTI) symptoms, contacts, patients with community-acquired pneumonia, elderly patients (>65 years old) with RTI, and medical staff. Additionally, citizens had the possibility to get either PCR or IgM/IgG testing in private medical centers without meeting these criteria. Local regulations complemented the federal orders. In Saint Petersburg, where the present study was performed, the Health Committee issued a ruling [3], which, among other requirements, established the form for the referral of clinic samples, which included data for RTI symptoms and previous contacts with infected subjects. These measures provide most of the data for estimating SARS-CoV-2 spread and seroprevalence in the population from the first month of the COVID-19 pandemic, including data for the epidemiologic status of examined persons.
Natural infection or vaccination are two sources of anti-COVID-19 antibodies, but neither provide long-term immunity [4]. Depending on population immunization and pathogen evolution, the COVID-19 pandemic spread in Russia can be divided into two periods. The period between March 2020 and January 2021 is considered the first phase of pandemic development [5]. Two increases in morbidity were identified in this time interval: (I) the spring-summer wave and (II) the autumn-winter wave [6]. Natural infection is the only source of immunity at this stage. The second stage of the COVID-19 pandemic in Russia (from February 2021 to the end of pandemic spread) began with the change in prevailing variants from Alpha to Delta and then Omicron variants. At the same time, on December 05, 2020, anti-SARS-CoV-2 vaccines became available in Russia [4]. In December 2020, anti-SARS-CoV-2 vaccination was included in the National Vaccination Schedule, but initially, the vaccine was available only for priority risk groups, like medical staff or service workers [7]. In Saint Petersburg, at six months after the start of immunization, only nearly 10% of citizens received two-dose vaccination [8]. Although in Russia there were several kinds of vaccines developed, the two-dose adenovirus vector-based Sputnik V has commonly (up to 95% of vaccinated population) been used for immunization [9]. Two other vaccines, EpiVacCorona (peptide-based) and CoviVac (inactivated virus), also were available at the beginning of immunization, but to a much lesser extent.
However, there were low COVID-19 vaccine acceptance rate in Russia, and in Saint Petersburg, the biggest city in Northwestern Russia only 32% of population were vaccinated untill Septembre 30, 2021 [10-12] . The regional governments resorted to restrictive measures against the unvaccinated population to increase vaccination. In Saint Petersburg vaccination became obligatory for several occupational groups [13], and from October 18, 2021, the Health Committee under the Saint Petersburg Government issued the ruling [14], that limited the access of unvaccinated subjects to community events, stores, and catering. Vaccination did not stop transmission, but it significantly reduced risks of severe disease or mortality in vaccinated subjects [15,16].
In November and December 2021, the Omicron variant emergence led to the maximal morbidity rate in the fifth period of the COVID-19 spread (10 January 2021–26 June 2022) [5]. This variant had multiple mutations in the spike protein [17] and could escape protective immunity, including the protective effect of Sputnik V immunization [18]. On the other hand, retrospective studies identified a decrease of anti-RBD titers in Sputnik V-vaccinated subjects on day 180 post-vaccination [18-20]. Revaccination provides a more stable protective effect [21].
The minimal proportion of persons with protective immunity in the population that prevents the infection transmission is considered the herd immunity threshold [22]. For the majority of infections, the presence of at least 60-80% immunopositive subjects protects the whole population from the infection spreading [22-25]. However, for the COVID pandemic, herd immunity could not be achieved because of waning of vaccine immunity and emergence of new strains more antigenically distant from the vaccine [26].
In Saint Petersburg, during the fifth wave, from 04 March 2022, most restrictions were lifted without the increase of morbidity till the next rise of infection spread in the end of summer. The aim of the present retrospective study was the estimation of population immunity in Saint Petersburg and neighboring regions in the context of a complex set of factors, including infection spread in 2020, vaccination in 2021 and Omicron spread in 2022. Additionally we analyzed associations between IgG levels and self-reported health or vaccination status in patients who underwent tests for anti-SARS-CoV-2 IgG in 2020–2022.
2.1. Study participants and settings
Anonymized data were collected in the Laboratory Information System (LIS) of the North-West Centre for Evidence-Based Medicine. The data for patients, who requested serological tests for anti-SARS-CoV-2 IgG from May 2020 to May 2024 in northwestern Russia were included in the analysis. All procedures were in accordance with the ethical standards of the institutional research committee and national standards. For this type of retrospective study, formal consent is not required.
2.2. Detection of anti-SARS-CoV-2 IgG
2.2.1 Enzyme-linked immunoassays (ELISA)
The Euroimmun Anti-SARS-CoV-2 ELISA IgG (Euroimmun, Germany) test kit was applied for semiquantitative anti-SARS-CoV-2 IgG levels estimation. In this assay, the entire S1 subunit SARS-CoV-2 spike glycoprotein is used as an antigen [27]. The assay procedure was automated using a HydroFlex microplate washer, Infinite F50 reader, and Magellan software (Tecan Group Ltd, Switzerland). The conversion Euroimmun IgG optical density ratios (ODR) in the range 0–8.5 was performed using the previously described non-linear formula [28], which was previously developet to improve matching between Euroimmun ELISA and SARS-CoV-2 IgG II Quant (Abbott, Ireland) CLIA ODR values were converted to Abbott AU/ml by the non-linear formula.
AU/ml = tan(ODR*π/18)/0.0009
and then converted to BAU/ml applying the conversion factor 0.142 as previously established by Abbott with the WHO international standard NIBSC 20–136 and described elsewhere [29,30]. The results that exceed this range were adjusted to 8.5 ODR units to receive the relevant conversion result.
2.2.2. Chemiluminescence immunoassay (CLIA)
Two CLIA-based assays were applied to estimate IgG serum levels, i.e., Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics GmbH, Switzerland) and SARS-CoV-2 IgG II Quant (Abbott, USA).
The Elecsys Anti-SARS-CoV-2 S assay applies the double antigen sandwich format for SARS-CoV-2 RBD-specific IgG detection. Measurement results vary in 0.40 — 250 U/mL interval. Results that fell below this range were adjusted to 0.4 U/mL. Using the conversion factor 1.216 which previously established by Roche with the WHO international standard NIBSC 20–136 [30], we recalculated the results in BAU/ml.
Estimating anti-RBD IgG serum levels in the other group of patients was done using the SARS-CoV-2 IgG II Quant (Abbott, Ireland) kit. The IgG levels were automatically examined, providing the results in Arbitrary Units per milliliter (AU/mL). The test-specific values in AU then were multiplied by the correction factor of 0.142, to reach the values for BAU/mL, following the manufacturer’s instructions and in accordance with conversion factor established by Abbott with the WHO international standard NIBSC 20–136.
2.3 Statistics
The Wilcoxon test was applied to evaluate the differences between patient groups. P values were adjusted based on Holm’s method; Padj<0.05 was considered statistically significant.
The z-test was applied to compare anti-SARS-CoV-2 seropositivity in different groups. P values were adjusted based on Holm’s method, and adjusted Padj<0.05 was considered statistically significant. The standard Wald confidence interval for proportions was estimated.
The R (version 4.3.2) package was used to perform all statistical analyses. The results were visualized with the R package ggplot2 16. [31].
3.1. Study population
The anti-SARS-CoV-2 IgG levels data for a total of 41,245 individuals were extracted from LIS. Table 1 describes the demographic characteristics and self-reported epidemiologic status (i.e., “Healthy”, “COVID” (PCR-confirmed cases, including convalescent subjects), “URTI” (upper respiratory tract infection, including convalescent subjects), or “Vaccinated”) of groups for all three tests. Epidemiologic status was available only for some samples.
3.2. Annual anti SARS-CoV-2 IgG levels and seroprevalence in Northwestern Russia in 2020–2024
All applied tests demonstrated the growth of median anti-SARS-CoV-2 IgG levels and seroprevalence in the study population with the progress of the COVID-19 pandemic (Fig 1a,Table 2). On the other hand, three assays demonstrated some disagreement, which may be explained by their technical differences. The anti-SARS-CoV-2 IgG levels identified by the Euroimmun Anti-SARS-CoV-2 ELISA IgG (Euroimmun, Germany) assay seem to be lower than levels identified by the CLIA tests. Also, the Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics GmbH, Switzerland) identifies the IgG levels in the limited range from 0.411 to 257.2 BAU/ml; instead, the SARS-CoV-2 IgG II Quant (Abbott, Ireland) assay permits quantifying a wider range of IgG levels.
Table 1. Demographic and epidemiological characteristics of the study population (N = 41,295 subjects]
| Diagnostic assay | Euroimmun Anti-SARS-CoV-2 ELISA IgG (Euroimmun, Germany) | Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics GmbH) | SARS-CoV-2 IgG II Quant (Abbott) |
| Period of study | May 2020-December2021 | March 2021-June 2024 | May 2021-May 2024 |
| n | 4408 | 14528 | 22359 |
| Year | |||
| 2020 | 1510 | 0 | 0 |
| 2021 | 2888 | 14311 | 16948 |
| 2022 | 10 | 183 | 5048 |
| 2023-2024* | 0 | 34 | 363 |
| Gender | |||
| Male | 1762 | 5306 | 8830 |
| Female | 2646 | 9220 | 13527 |
| Age (mean and SD) | 48.3 (15.74) | 52.5 (16.42) | 48.9 (16.14) |
| ?Percent of adult (adult (18 y.o. and older) | 97.10% | 98.90% | 98,1% |
| Clinical-epidemiological status (self-reported group)*. | |||
| Asymptomatic | 3143 | 10405 | 14919 |
| COVID | 476 | 2024 | 3729 |
| URTI | 378 | 1262 | 1891 |
| Vaccinated | 10 | 467 | 2591 |
*Epidemiologic status was available only for part of the study group.
Euroimmun Anti-SARS-CoV-2 ELISA IgG (Euroimmun, Germany) testing was initiated earlier than the CLIA test and finished in 2022 (only 10 subjects were examined in 2022). It demonstrates the significant growth of median anti-SARS-CoV-2 IgG levels and seroprevalence in 2021 compared to 2020 (Padj < 0.01, Figure 1a, Table 2).
The pairwise comparison of annual anti-SARS-CoV-2 IgG levels identified by SARS-CoV-2 IgG II Quant (Abbott, Ireland) demonstrated the over two-fold (Padj < 0.01, Figure 1a, Table 2) rise of BAU/ml values in 2022 compared to 2021. Additionally, there is a significant increase in anti-SARS-CoV-2 IgG levels in serum samples in 2023 compared to the previous year ( Padj < 0.01, Figure 1a, Table 2). On the other hand, the anti-SARS-CoV-2 IgG level in 2024 did not significantly increase compared to 2023. The anti-SARS-CoV-2 IgG seroprevalence identified with the SARS-CoV-2 IgG II Quant (Abbott, Ireland) test increased significantly from 47+/-0.01% in 2021 to 76+/-0.07 % in 2022. The further seroprevalence growth in 2022–2024 did not reach statistical significance (Figure 1b, Table 2).
The results of the Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics GmbH, Switzerland) assay are available for 2021-2024, and the identified IgG level growth in 2022 compared to 2021, and a slight augmentation of IgG level in 2023 compared to previous periods (Figure 1b, Table 2). There is a gradual increase in the proportion of people seropositive to SARS-CoV-2, from 58.8% in 2021 to almost 100% in 2024. Meanwhile, differences in seroprevalence from year to year did not reach statistical significance (Table 2).
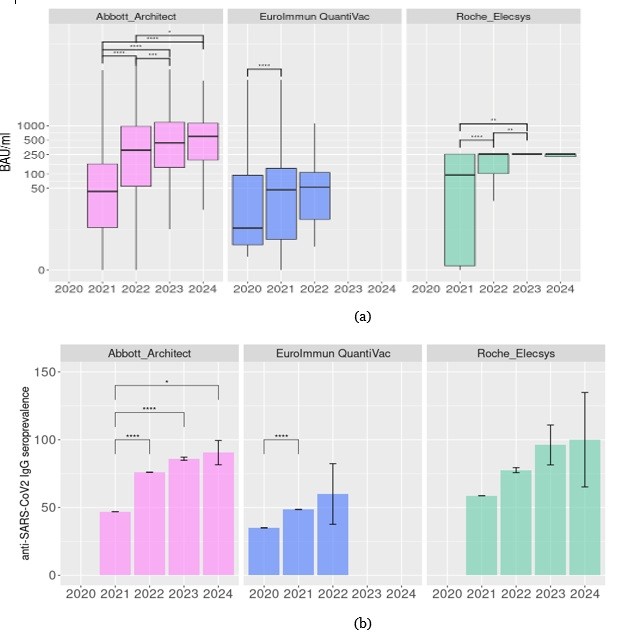
Figure 1. Retrospective analysis of anti SARS-CoV-2 IgG levels in Northwestern Russia in 2020–2024. (a) IgG levels identified in different years by the diverse assays (b) Frequency of anti SARS-CoV-2 IgG-positive cases in the population. *Padj< 0.05, **Padj< 0.01, ***Padj< 0.001, ****Padj< 0.0001.
Table 2. The characteristics of samples submitted for anti-SARS-CoV-2 IgG examination in different years included in the analysis
| Test | Year | IgG levels (BAU/ml) | Seropositive samples | ||
| median | IQR | % | SI | ||
| SARS-CoV-2 IgG II Quant (Abbott) | 2021 | 43 | 152.8 (7.5-160.3) | 47 | 0.01 |
| 2022 | 310.1 | 914.9 (54.8-969.7) | 76 | 0.07 | |
| 2023 | 438.4 | 1038.7 (134.9-1173.6) | 85.9 | 1.17 | |
| 2024 | 604.6 | 929.3 (194-1123.3) | 90.5 | 8.94 | |
| Euroimmun Anti-SARS-CoV-2 ELISA IgG | 2020 | 7.3 | 90.5 (3-93.5) | 35 | 0.1 |
| 2021 | 46.4 | 126.4 (4.1-130.5) | 48.5 | 0.07 | |
| 2022 | 52.8 | 93.2 (13.7-106.9) | 60 | 22.34 | |
| Elecsys Anti-SARS-CoV-2 S (Roche diagnostics) | 2021 | 94.6 | 256.8 (0.4-257.2) | 58.8 | 0.02 |
| 2022 | 257.2 | 156 (101.2-257.2) | 77.59 | 1.84 | |
| 2023 | 257.2 | 0 (257.2-257.2) | 96.2 | 14.71 | |
| 2024 | 257.2 | 26.5 (230.7-252.2) | 100 | 34.81 |
3.3. Seasonal dynamics of anti-SARS-CoV-2 IgG levels in population serum samples in Northwestern Russia in 2020–2024 and impact of initiation of vaccination program
Despite the year-by-year trend analysis of anti-SARS-CoV-2 IgG levels in the North-Western Russian population demonstrating sustained growth, the seasonal dynamics of population immunity seem to be more complex (Fig. 2a,b).
In 2020, the Euroimmun Anti-SARS-CoV-2 ELISA IgG (Euroimmun, Germany) assay demonstrated significant growth of anti-SARS-CoV-2 IgG levels in the population from spring to summer season (Padj < 0.01, Fig. 2a). Further observation did not find any significant increase in anti-SARS-CoV-2 levels in population serum samples from summer to autumn. Instead, in the winter of 2021 (including December 2020), the IgG levels in studied samples were considerably higher (Padj < 0.01, Fig. 2a) compared to the previous season. This increase may be associated with COVID spread [5] or the early vaccination program. After the winter of 2021, the levels of anti-SARS-CoV-2 IgG identified by the Euroimmun Anti-SARS-CoV-2 ELISA IgG (Euroimmun, Germany) assay remained stable with the non-significant tendency to decrease in the spring of 2021 and to grow in the autumn of 2021 after the initiation of mandatory vaccination for selected groups.
From the winter of 2021, the results of more sensitive CLIA assays, i.e., Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics GmbH, Switzerland) and SARS-CoV-2 IgG II Quant (Abbott, USA), became available. In contrast to the ELISA test, the results obtained by CLIA tests demonstrate the significant reduction in IgG levels in the population in spring 2021 compared to the previous winter (Padj<0.01, Fig. 2a) despite the ongoing vaccination program. Then Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics GmbH, Switzerland), but not SARS-CoV-2 IgG II Quant (Abbott, USA) detected a slight statistically significant rise (Padj<0.01, Fig. 2a) of IgG levels in studied samples in summer. Both CLIA assays identified an increase of IgG levels in serum samples studied in autumn 2021 compared to the previous month of the survey (Padj < 0.01, Fig. 2) that may mirror preceding COVID spread or growth of vaccination, or both. In the spring of 2022 (including December 2021), the levels of IgG identified by Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics GmbH, Switzerland) and SARS-CoV-2 IgG II Quant (Abbott, USA) demonstrated a further gain, which is significant (Padj<0.01, Fig. 2a) only for Abbott CLIA test results, possibly due to unprecedented Omicron spread [5].
The frequency of seropositive cases identified by CLIA also decreased in spring 2021 compared to the previous winter and elevated after the stimulation of the vaccination program in summer 2021. Then the seroprevalence stopped growwing and no longer depended on the season, i.e., no decreases were demonstrated in spring 2022 or summer 2022 compared to winter 2022 (Fig. 2b).
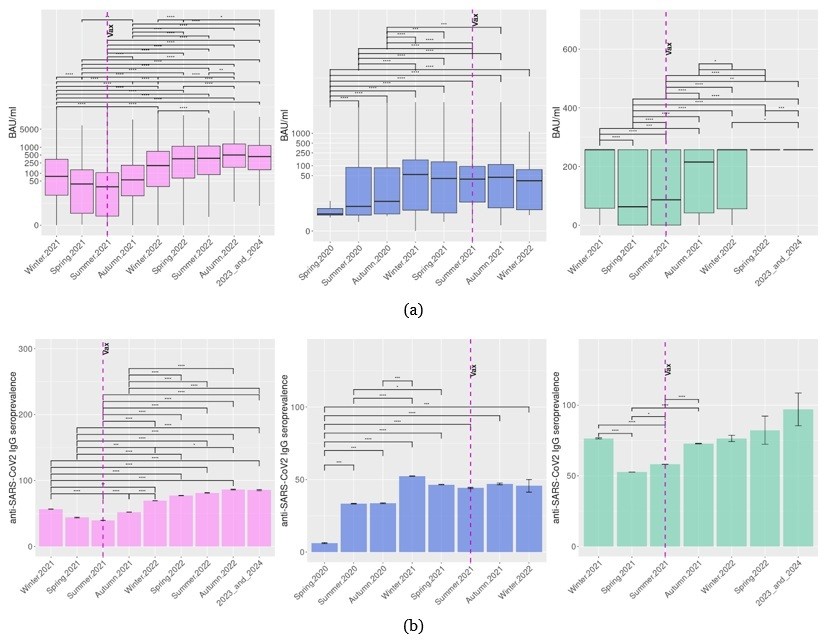
Figure 2. Seasonal variations of anti-SARS-CoV-2 IgG levels (a) and seropositivity (b) in 2020–2024 in Northwestern Russia. Program of mandatory vaccination in elected occupational groups in Russia in Summer 2021 is signed by a dotted line. *Padj< 0.05, **Padj< 0.01, ***Padj< 0.001, ****Padj< 0.0001. Liliac plots represent results for SARS-CoV-2 IgG II Quant (Abbott) test, blue for Euroimmun Anti-SARS-CoV-2 ELISA IgG (Euroimmun, Germany), and green for Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics GmbH) .
3.4. The anti-SARS-CoV-2 IgG testing demonstrated the effectivity of COVID-19 identification and vaccination program
At blood sampling, patients were asked about existing/recent URTI or COVID-19 disease or anti-SARS-CoV-2 vaccination (asymptomatic persons after contact with COVID-19-infected subjects were included in the asymptomatic group). In 2020, in asymptomatic subjects or subjects who suffered URTI, anti-SARS-CoV-2 IgG levels identified by the ELISA were significantly lower than in subjects who had a positive SARS-CoV-2 PCR test previously (Padj<0.01, Fig. 3a). Also, the seroprevalence was higher in the “COVID” group (72.9%) compared to the asymptomatic group (32.9%) and “URTI” (42.6%) group (Fig. 3a). However, IgG levels in URTI patients were higher compared to asymptomatic subjects (Padj<0.01, Fig. 3a). The same trend was identified by the ELISA test in 2021.
The CLIA testing results demonstrate the significantly higher anti-SARS-CoV-2 IgG levels and seroprevalence in COVID-19-suffered or vaccinated subjects compared to non-vaccinated asymptomatic and URTI-suffered subjects in 2021 (Padj< 0.01, Fig. 3a, b). However, in 2022, the Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics GmbH, Switzerland) test did not reveal any differences in the serum anti-SARS-CoV-2 antibody levels or seroprevalence between these groups. In the same period, the SARS-CoV-2 IgG II Quant (Abbott, Ireland) test identified higher IgG levels in COVID-19-suffering and vaccinated subjects but also did not reveal any differences in seroprevalence between groups. It should be noted that in this period, subjects may place themselves in the most relevant of the proposed epidemiological groups at the moment, despite previous COVID-19 or vaccination.
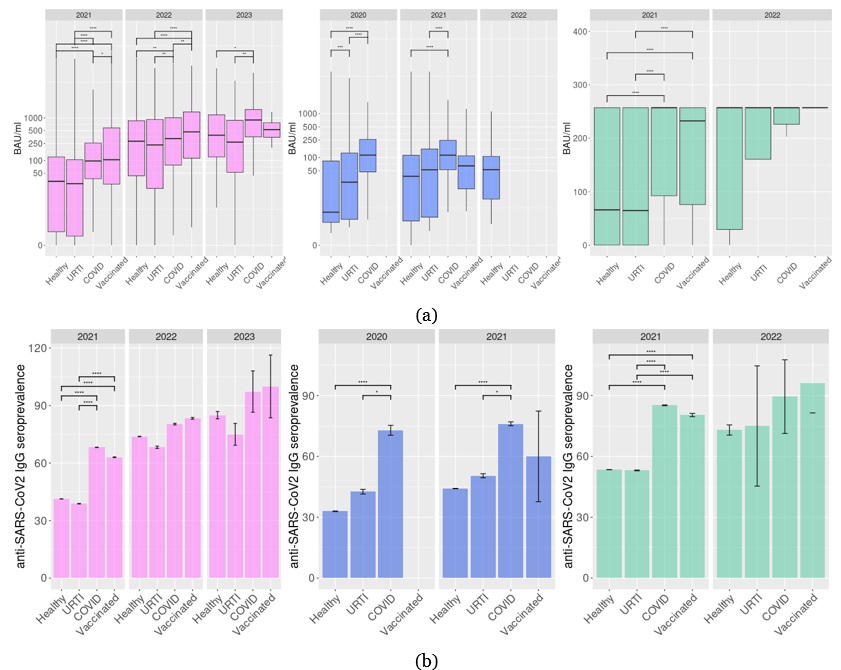
Figure 3. Anti-SARS-CoV-2 IgG levels (a) and seropositivity (b) in 2020–2024 in subjects who were examined after COVID-19, URTI or anti SARS-CoV-2 vaccination. *Padj< 0.05, **Padj< 0.01, ***Padj< 0.001, ****Padj< 0.0001. Liliac plots represent results for SARS-CoV-2 IgG II Quant (Abbott) test, blue for Euroimmun Anti-SARS-CoV-2 ELISA IgG (Euroimmun, Germany), and green for Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics GmbH).
The WHO declared the end of the pandemic phase of COVID‐19 on May 5, 2023. However, the retrospective analysis of anti-SARS-CoV-2 immunity, taking into account the government’s measures to control and prevent the disease, may be useful to understand their effectiveness. Immunity in the population includes both vaccinated persons and subjects who had SARS-CoV-2 infection [32,33]. To describe the immune status of the Northwestern Russian population in the context of the infection spread and preventive measures introduction, we analyzed the data of routine anti-SARS-CoV-2 IgG testing, including IgG levels and seropositivity, which are considered the relevant characteristics of herd immunity [34,35]. Our study population included residents of St. Petersburg and from the Leningrad, Novgorod, and Kaliningrad regions, who were mostly from small towns. In such a population, SARS-CoV-2 infection spread between closely interacting subjects should have been restricted by the immune layer and low probability of contacts with susceptible persons [30].
The analysis of anti-SARS-CoV-2 IgG levels and seropositivity allows us to demonstrate a gradual growth in both values in 2020–2022. The herd immunity threshold for initial SARS-CoV-2 strains, Alpha, and Delta strains was considered to be 60–70%, 75–80%, and 80%, respectively [25,36]. Our data confirm that in 2022 population immunity in the Northwestern Russian population reached this threshold. In the follow-up, in 2023–2024, the seropositivity level exceeded 90% [5].
Modelling of immune response fading following vaccination demonstrated that over a period of 250 days, there is a rapid, up to 50%, decline in antibody neutralizing activity, especially in individuals without natural immunity [37]. Comparing the long-term efficacy of adenoviral vector-based vaccines (including Sputnik V) and mRNA-based vaccines, it turns out that adenoviral vaccines provide a slower dynamic of the primary growth of antibody levels with a more pronounced maintenance of neutralizing titers in an 8-month follow-up. The putative mechanism of this phenomenon includes differences in antibody-depend. The putative mechanism of this phenomenon includes differences in antibody-dependent cellular phagocytosis and antibody-dependent complement deposition [38]. On the other hand, the impact of vaccination on the development of public immunity is mainly manifested in a decrease in the basic reproduction number (R0) [39]. Despite the undoubted benefit, only a decrease in R0 does not completely eliminate the threat of the spread of new virus strains, which maintains the risk of an epidemic in the future. Simultaneously, notable reduction in Sputnik V efficacy against the new SARS-CoV-2 variants like B.1.1.529 (Omicron BA.5) was described [40].
The seasonal dynamic of anti-SARS-CoV-2 IgG levels did not demonstrate gradual growth. In the first year of the pandemic, from spring 2020 to winter 2020–2021, median IgG levels in the population increased. However, in spring 2021, we revealed a decrease both in IgG median levels and seroprevalence[5], despite the initiated vaccination program. It could also reflect a slowdown in the spread of the infection [5], including as a result of early vaccination program, although the number of people vaccinated was amounting to about 10% of the population [41]. Also, previously it was demonstrated that in 6 months post-infection, IgG avidity grows, but the levels of antibodies decline [42]. In summer 2021, slight growth of median IgG levels and seropositivity was identified by the Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics GmbH) test, but not by the other two assays. However, in the following autumn, both CLIA tests demonstrated a significant growth of both values. The increase identified by Euroimmun Anti-SARS-CoV-2 ELISA IgG (Euroimmun, Germany) remained insignificant, possibly due to an insufficient number of samples. Simultaneously, in the summer of 2021, the spread of the Delta variant was accompanied by a significant increase in COVID-19 incidence and hospitalizations [5]. At the same time, there was tightening of the requirements for vaccination in several professional groups [13]. Both factors may have contributed to increased seroprevalence in autumn 2021 compared to the previous season. In November 2021, several restrictions for the unvaccinated subjects increased vaccination again [14], and Delta spread has slowed down [5]. However, in the following winter, in January 2022, the Omicron lineage spread in the Northwestern population. In Saint Petersburg, the morbidity exceeded mean levels in Russia and reached 12,200 cases per 100,000 [6,43]. Against that backdrop, anti-SARS-CoV-2 IgG median levels and seroprevalence reached maximal values. Massive vaccination in Northwestern Russia population in 2022 made it possible to abandon strict quarantine measures and other restrictions. Meanwhile, despite all public health efforts, the rapid SARS-CoV-2 evolution and the emergence of new lineages caused new outbreaks of COVID-19 in 2022-2023 [26].
Additionally, we estimated median anti-SARS-CoV-2 IgG levels and seroprevalence in different groups, in accordance with self-reported health status. In 2020–2021, subjects who reported themself as asymptomatic or URTI groups had both values significantly lower than subjects who self-reported as COVID-suffering (or COVID-suffered). Interestingly, in 2020 there were slightly higher median IgG levels in URTI group compared to the asymptomatic group, but in 2021 and in following years this was no longer the case. This may mirror the improvement of SARS-CoV-2 RT-PCR testing.
High vaccination rates prevented the COVID pandemic from spreading and contributed significantly to seroprevalence [44]. The comparison of anti-SARS-CoV-2 IgG levels in COVID-group and vaccinated group revealed slightly higher median IgG levels in the vaccinated population in samples tested with SARS-CoV-2 IgG II Quant (Abbott, Ireland), but not other tests. In 2021, COVID and vaccinated groups demonstrated higher median IgG and seropositivity than the other two epidemiologic groups in our study. In 2022 the difference between groups became apparent.
The results of the present study are subject to some limitations. We did not take into account the subjects' medical state and age, so our analysis may be prone to bias [45]. Also, all data about vaccination or respiratory infections were self-reported. Simultaneously, we have had to apply data accepted with different test systems based on distinct methods (ELISA, CLIA) and providing discrepant results despite the standardization as identified previously [46]. Also, we had less data for 2023–2024. Additionally, we had no data for the anti-nucleocapsid IgG testing, which distinguishes vaccinated and naturally infected subjects, in our study population because this test was not performed routinely in the Nucleocapsid IgG testing and was not performed in the North-West Centre for Evidence-Based Medicine. Also, the healthcare specialists could apply only medical devices that were registered in Russia for in vitro diagnosis. When the patients were studied for anti-SARS-CoV-2 IgG, only anti-spike IgG testing was approved for screening by the Federal Service for Surveillance in Healthcare.
Despite all limitations described above, we revealed similar trends by different diagnostic systems. We identified a gradual increase of median anti-SARS-CoV-2 IgG levels and seroprevalence in the studied population in 2020–2022, which reached maximal values in 2022 and retained in follow-up. The available data also indirectly demonstrate the low effect of vaccination initiation in the incredulous population of North-Western Russia district till the stimulating measures were introduced.
The current study reports the dynamic seroprevalence of SARS-CoV-2 in the North-Western Russia federal district in the COVID pandemic from the spring of 2020 to May 2024. At the beginning of the COVID-19 pandemic, before the introduction of the vaccination, the only control methosds were restrictive measures [34], and median IgG levels in the population demonstrated a trend of decreasing when morbidity became lower in spring 2021. The highest prevalence of anti-SARS-CoV-2 IgG antibodies was achieved only after measures to increase vaccination and unprecedented spread of Omicron lineages. Vaccination program contributed to seropositivity in the studied population; however, the effect of the dramatic Omicron spread in the winter of 2022 on population immunity growth had a large effect on seroprevalence in the Northwestern Russian population.
All data underlying the results are available as part of the article and no additional source data are required.
The authors acknowledge Saint-Petersburg State University for a research grant 129659216.
Conceptualisation, AVI, EVS, and ANV; methodology, ANV; software, ANV; validation, AVI; formal analysis, ANV; investigation, ANV, EVS, and AVI; resources, AVI; data curation, ANV; writing—original draft preparation, EVS and ANV; writing—review and editing, AVI and ANV; visualisation, ANV; supervision, AVI; project administration, AVI; funding acquisition, ANV. All authors have read and agreed to the published version of the manuscript.
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
1.About temporary procedure for the organization of work of the medical organizations for the purpose of implementation of measures for prevention and decrease in risks of spread of new coronavirus infection COVID-19 [Internet]. Russian Ministry of Health; 2020. Available from: https://minzdrav.gov.ru/documents/9746-prikaz-ministerstva-zdravoohraneniya-rossiyskoy-federatsii-ot-19-marta-2020-g-198n-red-ot-22-12-2022-o-vremennom-poryadke-organizatsii-raboty-meditsinskih-organizatsiy-v-tselyah-realizatsii-mer-po-profilaktike-i-snizheniyu-riskov-rasprostraneniya-novoy-koronavirusnoy-infektsii-covid-19.
2.On additional measures aimed at reducing risks of COVID-19 spread [Internet]. Chief State Sanitary Doctor of the Russian Federation; 2020. Available from: http://publication.pravo.gov.ru/Document/View/0001202004010005.
3.On amending to Saint Petersbourg Government Ruling № 121 from 13 March 2020 . Health committee under the Saint Petersburg Government; 2020. Available from: http://zdrav.spb.ru/media/filebrowser/%D0%BE%D1%82_13.03.20_121.pdf.
4.Uusküla A, Pisarev H, Tisler A, Meister T, Suija K, Huik K, et al. Risk of SARS-CoV-2 infection and hospitalization in individuals with natural, vaccine-induced and hybrid immunity: a retrospective population-based cohort study from Estonia. Sci Rep. 2023 Nov 21;13(1):20347. Available from: https://doi.org/10.1038/s41598-023-47043-6..
5.Akimkin V, Semenenko TA, Ugleva SV, Dubodelov DV, Khafizov K. COVID-19 Epidemic Process and Evolution of SARS-CoV-2 Genetic Variants in the Russian Federation. Microbiology Research. 2024 Mar;15(1):213–24. Available from: https://doi.org/10.3390/microbiolres15010015..
6.Karpova LS, Stolyarov KA, Popovtseva NM, Stolyarova TP, Danilenko DM. Comparison of the First Three Waves of the COVID-19 Pandemic in Russia in 2020–21. Epidemiology and Vaccinal Prevention. 2022 May 10;21(2):4-¬16. Available from: https://doi.org/10.31631/2073-3046-2022-21-2-4-16..
7.Prisyazhnyuk YP, Stperanskaya YS. Citizens' rights to immunoprophylaxis during the COVID-19 pandemic (in the context of the World Health Organization's recommendations). Russian Law Journal. 2020;4(5):44–8. Available from: https://elibrary.ru/item.asp?id=46560420..
8.Information sheet on cointeraction to the COVID pandemic [Internet]. Saint Petersburg Governement press center; 2021. Available from: https://www.gov.spb.ru/press/government/216361/..
9.Mazin PV, Hafizjanova RF, Egorova AI, Arjaeva MM. Transfer-factor and anti-COVID-19 vaccines. National researchers association. 2023;(89–3):9–16. Available from: https://doi.org/: 10.31618/NAS.2413-5291.2023.3.89.758 .
10.Tran VD, Pak TV, Gribkova EI, Galkina GA, Loskutova EE, Dorofeeva VV, et al. Determinants of COVID-19 vaccine acceptance in a high infection-rate country: a cross-sectional study in Russia. Pharm Pract (Granada). 2021;19(1):2276. Available from: https://doi.org/: 10.18549/PharmPract.2021.1.2276. .
11.Vaccination coverage in Saint Petersburg [Internet]. Government of Saint Petersburg.; 2021. Available from: https://www.gov.spb.ru/press/governor/222991/. .
12.Sallam M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines. 2021 Feb;9(2):160. Available from: https://doi.org/: 10.3390/vaccines9020160. .
13.On amending to Saint Petersbourg Government Ruling № 121 from 13 March 2020 (July 30, 2021) [Internet]. Health committee under the Saint Petersburg Government; 2021. Available from: https://rg.ru/documents/2021/07/30/spb-post549-reg-dok.html. .
14.On amending to Saint Petersbourg Government Ruling № 121 from 13 March 2020 (October 18, 2021). Health committee under the Saint Petersburg Government; 2021. Available from: http://publication.pravo.gov.ru/document/view/7800202110250001. .
15.Shkoda AS, Gushchin VA, Ogarkova DA, Stavitskaya SV, Orlova OE, Kuznetsova NA, et al. Sputnik V Effectiveness against Hospitalization with COVID-19 during Omicron Dominance. Vaccines. 2022 Jun;10(6):938. Available from:https://doi.org/:10.3390/vaccines10060938. .
16.Gladkikh A, Dedkov V, Sharova A, Klyuchnikova E, Sbarzaglia V, Kanaeva O, et al. Epidemiological Features of COVID-19 in Northwest Russia in 2021. Viruses. 2022 May;14(5):931. Available from: https://doi.org/:10.3390/v14050931. .
17.Willett BJ, Grove J, MacLean OA, Wilkie C, De Lorenzo G, Furnon W, et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat Microbiol. 2022 Aug;7(8):1161–79. Available from: https://doi.org/: 10.1038/s41564-022-01143-7. .
18.Radion EI, Mukhin VE, Kholodova AV, Vladimirov IS, Alsaeva DY, Zhdanova AS, et al. Functional Characteristics of Serum Anti-SARS-CoV-2 Antibodies against Delta and Omicron Variants after Vaccination with Sputnik V. Viruses. 2023 Jun;15(6):1349. Available from:https://doi.org/:10.3390/v15061349. .
19.Chahla RE, Tomas-Grau RH, Cazorla SI, Ploper D, Pingitore EV, López MA, et al. Long-term analysis of antibodies elicited by SPUTNIK V: A prospective cohort study in Tucumán, Argentina. The Lancet Regional Health – Americas [Internet]. 2022 Feb 1 [cited 2025 May 9];6. Available from: https://www.thelancet.com/journals/lanam/article/PIIS2667-193X(21)00119-8/fulltext. .
20.Sanchez L, Rouco SO, Pifano M, Ojeda DS, Pascuale CA, Mazzitelli B, et al. Antibody durability at 1 year after Sputnik V vaccination. The Lancet Infectious Diseases. 2022 May 1;22(5):589–90. Available from: https://doi.org/:10.1016/S1473-3099(22)00176-1. .
21.Drapkina OM, Berns SA, Chashchin MG, Gorshkov AY, Zhdanova OV, Ryzhakova LN. Severity and duration of immune response in people of different age categories after SARS-CoV-2 revaccination. Cardiovascular Therapy and Prevention. 2024 Feb 8;22(12):3870. Available from: https://doi.org/10.15829/1728-88002023-3870. .
22.Clemente-Suárez VJ, Hormeño-Holgado A, Jiménez M, Benitez-Agudelo JC, Navarro-Jiménez E, Perez-Palencia N, et al. Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic. Vaccines (Basel). 2020 May 19;8(2):236. Available from: https://doi.org/:10.3390/vaccines8020236. .
23.Randolph HE, Barreiro LB. Herd Immunity: Understanding COVID-19. Immunity. 2020 May 19;52(5):737–41. Available from: https://doi.org/:10.1016/j.immuni.2020.04.012. .
24.Schaeffer B, Taylor B, Bushman M, Hanage WP. The devil in the details: Herd immunity and pandemic response. Cell Host Microbe. 2021 Jul 14;29(7):1048–51. Available from: https://doi.org/:10.1016/j.chom.2021.06.017. .
25.Arbel Y, Arbel Y, Kerner A, Kerner M. Can COVID-19 herd immunity be achieved at a city level? PLoS One. 2024;19(5):e0299574.Available from: https://doi.org/:10.1371/journal.pone.0299574. .
26.Albright C, Van Egeren D, Thakur A, Chakravarty A, White LF, Stoddard M. Antibody escape, the risk of serotype formation, and rapid immune waning: Modeling the implications of SARS-CoV-2 immune evasion. PLoS One. 2023;18(10):e0292099. Available from: https://doi.org/:10.1371/journal.pone.0292099. .
27.Beavis KG, Matushek SM, Abeleda APF, Bethel C, Hunt C, Gillen S, et al. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for detection of IgA and IgG antibodies. Journal of Clinical Virology. 2020 Aug 1;129:104468. Available from: https://doi.org/:10.1016/j.jcv.2020.104468. .
28.Ivanov A, Kryshen E, Semenova E. Nonlinear interdependence of the results of measuring anti-SARS-CoV-2 IgG levels using Abbott and Euroimmun test systems. J Clin Virol. 2023 Jul;164:105448. Available from: https://doi.org/:10.1016/j.jcv.2023.105448. .
29.Müller L, Kannenberg J, Biemann R, Hönemann M, Ackermann G, Jassoy C. Comparison of the measured values of quantitative SARS-CoV-2 spike antibody assays. J Clin Virol. 2022 Oct;155:105269. Available from: https://doi.org/:10.1016/j.jcv.2022.105269. .
30.Infantino M, Pieri M, Nuccetelli M, Grossi V, Lari B, Tomassetti F, et al. The WHO International Standard for COVID-19 serological tests: towards harmonization of anti-spike assays. Int Immunopharmacol. 2021 Nov;100:108095. Available from: https://doi.org/:10.1016/j.intimp.2021.108095. .
31.Wickham H. ggplot2: Elegant Graphics for Data Analysis [Internet]. Springer-Verlag New York; 2016. Available from: https://ggplot2.tidyverse.org .
32.Qu L, Xie C, Qiu M, Yi L, Liu Z, Zou L, et al. Characterizing Infections in Two Epidemic Waves of SARS-CoV-2 Omicron Variants: A Cohort Study in Guangzhou, China. Viruses. 2024 Apr 22;16(4):649. Available from: https://doi.org/:10.3390/v16040649. .
33.Murphy TJ, Swail H, Jain J, Anderson M, Awadalla P, Behl L, et al. The evolution of SARS-CoV-2 seroprevalence in Canada: a time-series study, 2020–2023. CMAJ. 2023 Aug 14;195(31):E1030–7. Available from: https://doi.org/:10.1503/cmaj.230249. .
34.Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020 Jun 16;52(6):971-977.e3. Available from: https://doi.org/:10.1016/j.immuni.2020.04.023. .
35.Iyer AS, Jones FK, Nodoushani A, Kelly M, Becker M, Slater D, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020 Oct 8;5(52):eabe0367. Available from: https://doi.org/:10.1126/sciimmunol.abe0367. .
36.Gaviria A, Tamayo-Trujillo R, Paz-Cruz E, Cadena-Ullauri S, Guevara-Ramírez P, Ruiz-Pozo VA, et al. Assessment of the COVID-19 pandemic progression in Ecuador through seroprevalence analysis of anti-SARS-CoV-2 IgG/IgM antibodies in blood donors. Front Cell Infect Microbiol. 2024;14:1373450. Available from: https://doi.org/:10.3389/fcimb.2024.1373450..
37.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021 Jul;27(7):1205–11. Available from: https://doi.org/:10.1038/s41591-021-01377-8. .
38.Collier ARY, Yu J, McMahan K, Liu J, Chandrashekar A, Maron JS, et al. Differential Kinetics of Immune Responses Elicited by Covid-19 Vaccines. N Engl J Med. 2021 Nov 18;385(21):2010–2. Available from: https://doi.org/:10.1056/NEJMc2115596. .
39.Al-Arydah M. Assessing vaccine efficacy for infectious diseases with variable immunity using a mathematical model. Sci Rep. 2024 Aug 10;14(1):18572. Available from: https://doi.org/:10.1038/s41598-024-69651-6. .
40.Dolzhikova IV, Tukhvatulin AI, Grousova DM, Zorkov ID, Komyakova ME, Ilyukhina AA, et al. Immunogenicity and Protectivity of Sputnik V Vaccine in hACE2-Transgenic Mice against Homologous and Heterologous SARS-CoV-2 Lineages Including Far-Distanced Omicron BA.5. Vaccines. 2024 Oct;12(10):1152. Available from: https://doi.org/: 10.3390/vaccines12101152. .
41.St. Petersburg residents allowed to choose a vaccine for COVID-19 immunization [Internet]. Saint Petersburg TV. 2021 [cited 2025 Aug 19]. Available from: https://tvspb.ru/news/2021/05/29/peterburzhcam-razreshili-vybirat-preparat-dlya-vakcinacii-ot-covid-19. .
42.Löfström E, Eringfält A, Kötz A, Wickbom F, Tham J, Lingman M, et al. Dynamics of IgG-avidity and antibody levels after Covid-19. Journal of Clinical Virology. 2021 Nov 1;144:104986. Available from: https://doi.org/:10.1016/j.jcv.2021.104986. .
43.Klink GV, Danilenko D, Komissarov AB, Yolshin N, Shneider O, Shcherbak S, et al. An Early SARS-CoV-2 Omicron Outbreak in a Dormitory in Saint Petersburg, Russia. Viruses. 2023 Jun 22;15(7):1415. Available from: https://doi.org/:10.3390/v15071415. .
44.Yuan R, Chen ,Huimin, Yi ,Lina, Li ,Xinxin, Hu ,Ximing, Li ,Xing, et al. Enhanced immunity against SARS-CoV-2 in returning Chinese individuals. Human Vaccines and Immunotherapeutics. 2024 Dec 31;20(1):2300208. Available from: https://doi.org/:10.1080/21645515.2023.2300208 .
45.Pant B, Gumel AB. Mathematical assessment of the roles of age heterogeneity and vaccination on the dynamics and control of SARS-CoV-2. Infect Dis Model. 2024 Sep;9(3):828–74. Available from: https://doi.org/:10.1016/j.idm.2024.04.007. .
46.Chan A, Martinez-Cajas J, Yip PM, Kulasingam V, Garland J, Holland D, et al. Evaluation and comparison of four quantitative SARS-CoV-2 serological assays in COVID-19 patients and immunized healthy individuals, cancer patients, and patients with immunosuppressive therapy. Clin Biochem. 2023 Jun;116:1–6. Available from: https://doi.org/:10.1016/j.clinbiochem.2023.02.010. .