In today's world, biomedical innovations play a key role in the development of medicine and improving the quality of life. Progress in genetic research, development of digital medical technologies and the use of artificial intelligence in healthcare are opening up new opportunities for diagnosing, treating, and preventing diseases. However, along with unlimited prospects, these innovations raise a number of important issues related to the protection of constitutional rights and freedoms, including the right to privacy, physical and mental integrity and access to healthcare services. Considering the fact that human rights are the core of modern legal doctrine, the development of technology is aimed at ensuring a decent standard of living and health of people. Humanism is reflected in law as one of its key and inherent characteristics, reflecting the so-called spiritual aspect of law. Law serves as a bridge that transfers the ideals of humanism from the social and ethical domains to the legal sphere, thereby transforming humanity into a legal concept [1]. Even though the right to health is a part of general human rights standards, there are no specialised international legal mechanisms for the protection of biomedical rights [2].
However, the development of biomedicine has two sides. On the one hand, it opens up new opportunities for humanity to improve health, prolong life, and cure diseases that were previously considered incurable. New medical technologies embody the ideals of humanism, striving to make everyone's life better. On the other hand, the development of biomedicine raises complex ethical, social and legal issues. For example, access to advanced medical technologies is not equal for all the population, which violates the principles of social justice and equality. In addition, genetic research and the possibility of interfering with the human genome calls into question the right to genetic integrity and privacy, as information about a person's genetic code can be used not only for treatment but also for discrimination. Another issue arises with the development of artificial intelligence in medicine. The use of algorithms for diagnosis and treatment can increase the efficiency of medical care, but it also raises questions about the transparency of decisions, responsibility for medical errors, and the possibility of replacing human contact between doctor and patient with technologies.
Thus, while biomedical innovations have the potential to significantly improve the quality of life, they also require improved legal frameworks and ethical standards to ensure that fundamental rights and freedoms are protected in the new medical context. Hence, an important task for modern society is to find a balance between biomedical innovation and human rights protection to ensure that technological progress serves the benefit of all without exception. Accordingly, the ECtHR plays a key role in protecting and balancing the rights and freedoms of individuals in the context of rapid technological progress. The ECtHR’s task is to adapt existing human rights norms to the new challenges posed by biomedical progress. The Court does this through the interpretation of the European Convention on Human Rights [3], ensuring that the rights to privacy, information security, and physical integrity are protected in the context of medical innovations [4].
It is also worth noting that through decisions in specific cases, the ECtHR addresses issues related to ethical dilemmas and legal conflicts arising from biomedical innovations. The cases may concern the confidentiality of medical information, the right to genetic integrity, and access to healthcare services [5]. ECtHR judgments form case law that serves as a guide for national courts and legislators in the Council of Europe member states. ECHR judgments stimulate legislative and regulatory reforms aimed at ensuring better protection of human rights in the context of medical innovations.
The interplay between biomedical advancements and human rights has received significant scholarly attention, reflecting the ethical, legal, and societal challenges posed by emerging technologies. This literature review examines key works that inform the study of the ECtHR role in addressing these issues. Hendel [2] provides foundational insights into the role of the ECtHR in adjudicating cases that intersect with biomedical innovations. By analysing judgments involving genetic research, artificial reproduction, and medical ethics, Hendel [2] highlights the Court’s role in defining the contours of biomedical rights and their alignment with broader human rights frameworks. Similarly, Mikhalov [6] discusses the evolving nature of medical and genetic rights, emphasising the need for robust international legal mechanisms to address the challenges posed by biomedicine. These works underscore the growing complexity of ensuring human rights protections in the face of rapid technological progress.
Krushelnytska [7] provides up-to-date information on the role of the Convention on Human Rights and Biomedicine [8] and the protection of human rights in the context of biomedicine in selected European countries. The study examines how the ratification of the 1997 Convention influenced the development of national legislation in countries such as Sweden, Denmark, Iceland, Norway and Finland. Particular attention is paid to the analysis of legislative acts that establish rules for the protection of personal and physical integrity of persons in the context of the use of new biomedical technologies, including the possibility of interference with life, health, and human genetic material.
Ostrovska [9] examines the emergence of bioethics as a new ethical field resulting from the scientific and technological advancements in biology and medicine in the latter half of the 20th century and its subsequent incorporation into the international legal framework, particularly in the realm of human rights, through the leadership of UNESCO and the Council of Europe. The significance of the Convention on Human Rights and Biomedicine [8] is highlighted, laying the groundwork for the development of bioethics as a component of international law in the human rights sector. The study explores the role of soft law norms as a crucial source of international law in addressing contemporary bioethical issues, especially regarding human rights and biomedicine.
European scholars such as Prainsack and Buyx [10] delve into the concept of solidarity in biomedicine, discussing how social justice principles can guide equitable access to innovations. Drooghenbroeck and Rizcallah [5] delve into the jurisprudence of the ECtHR, exploring how its judgments interpret fundamental rights in biomedical contexts. Their analysis identifies patterns in the Court's reasoning, particularly regarding privacy, informed consent, and equitable access to healthcare. Rendtorff and Kemp [11] emphasise the ethical principles that underpin European bioethics, such as autonomy, dignity, and vulnerability. Their work explores the implications of these principles in biomedical decision-making, particularly in contexts like genetic editing and end-of-life care.
The intersection of ethics and law is further explored by Andorn [12], who defends the universal applicability of the UNESCO Declaration on Bioethics and Human Rights, arguing that it serves as a global standard for balancing scientific freedom with ethical responsibility. Gostin and Wiley [13] focus on public health law and its intersection with biomedical rights, advocating for a balance between innovation and equitable access. Both scholars highlight the necessity of robust legal frameworks to address the ethical dilemmas posed by advancements in medical technologies.
Yamnenko and Litvinova [14] discuss the challenges of ensuring data privacy in healthcare settings. These works emphasise the need for targeted legal and ethical safeguards to protect vulnerable groups from exploitation or discrimination in biomedical contexts. Several studies examine how ECtHR judgments influence domestic legal systems. Tarasevych, Yuzko, Hrabovska, Romanova and Lisova [4] argue that the Court’s decisions often catalyse legislative reforms, unifying national laws with international human rights standards. This dynamic is particularly evident in cases involving assisted reproduction, genetic privacy, and access to experimental treatments.
The existing literature reveals a consensus on the transformative potential of biomedical innovations and the corresponding need for legal and ethical safeguards. While international legal instruments and ECtHR judgments provide valuable frameworks, gaps remain in addressing emerging challenges, such as AI-driven diagnostics and the equitable distribution of advanced medical technologies. The reviewed works collectively underscore the importance of a multidisciplinary approach, integrating legal, ethical, and scientific perspectives to ensure that biomedical progress enhances, rather than undermines, human rights. In this regard, this research aims to examine the role of the ECtHR in addressing and resolving human rights issues arising from advancements in biomedicine. The research aim stipulates the following objectives:
This study adopts a multidisciplinary approach to examine the role of the ECtHR in protecting fundamental human rights in the realm of biomedical innovations. The methodology combines established legal methods and complementary analytical tools to comprehensively explore the intersection of law, ethics, and technological advancements. The formal legal method is central to this research, allowing for an in-depth analysis of legal texts, such as the European Convention on Human Rights [3] and the Convention on Human Rights and Biomedicine [8]. This method was essential in dissecting legal provisions, identifying rights relevant to biomedical advancements, and understanding their judicial application. Moreover, the comparative method was employed to juxtapose ECtHR case law with domestic legal frameworks of Council of Europe member states. This comparison highlighted differences and synergies in how various jurisdictions interpret and apply international human rights standards to biomedical issues.
Furthermore, to contextualise the evolution of legal protection for human rights in the biomedical field, the historical-legal method was used. This method traced the development of international bioethical principles and their incorporation into regional legal systems, providing insights into how legal responses have adapted to advancements in medical technology.
The doctrinal legal method focused on analysing judicial decisions and academic commentaries to understand the principles and reasoning underpinning ECtHR judgments. By examining legal scholarship and doctrinal interpretations, the study captured the broader theoretical underpinnings of the Court's decisions in biomedicine-related cases.
The interpretative method was utilised to explore how the ECtHR interprets provisions of the European Convention on Human Rights [3] considering emerging challenges in biomedicine. This approach helped uncover how the Court balances competing rights and interests, such as the right to privacy versus the right to health, in its judgments.
The functional legal method was applied to assess the practical impact of ECtHR judgments on national legislation and biomedical practices. This approach examined how the Court's decisions influence legislative reforms, regulatory policies, and institutional practices in member states.
Given the ethical complexities surrounding biomedical innovations, the ethical-legal method was incorporated to examine how ethical principles, such as autonomy, beneficence, and justice, are embedded within legal frameworks and ECtHR jurisprudence.
The critical legal method allowed for a critical examination of potential gaps or inconsistencies in the legal frameworks governing biomedical innovations. It also considered how power dynamics and socio-economic inequalities influence the accessibility and fairness of these advancements.
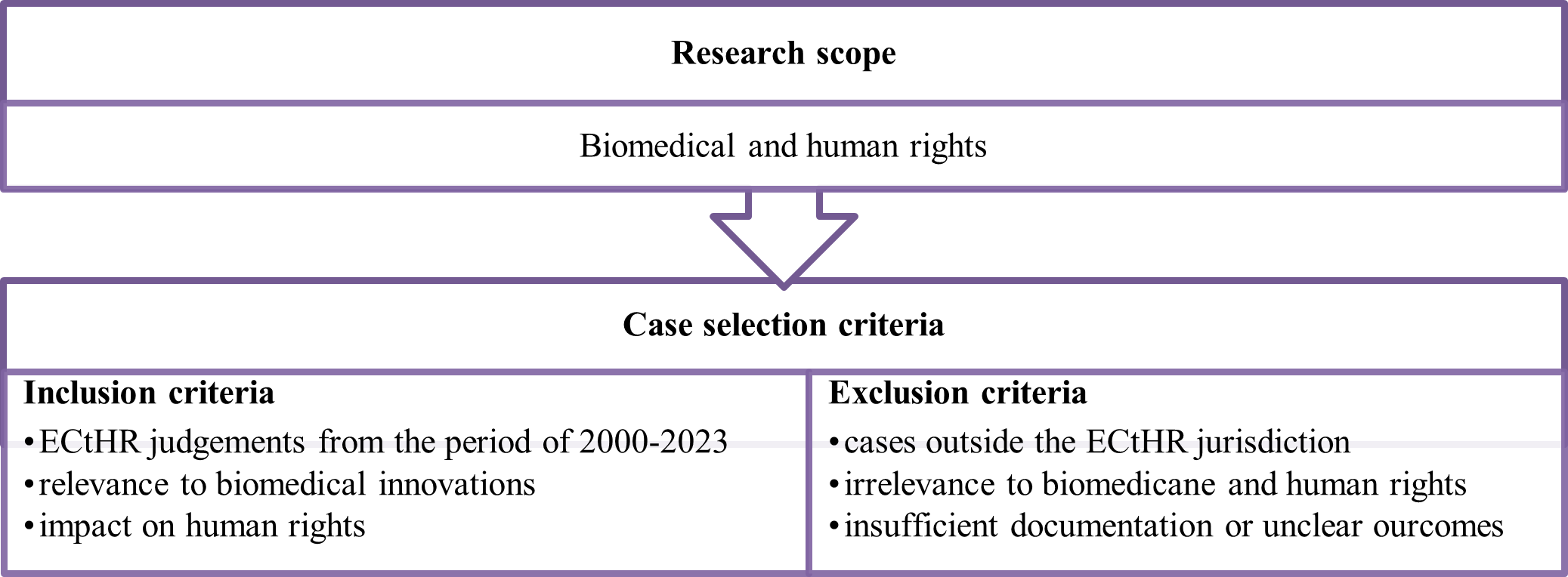
In addition, the study conducted a detailed analysis of significant ECtHR cases, including but not limited to: ‘Glass v. the United Kingdom’ [15], ‘C v. Italy’ [16], ‘G.M. and Others v. the Republic of Moldova’ [17]. The analysis explored how these cases shape legal norms and principles in the biomedical domain, focusing on issues such as informed consent, privacy, and equitable access to healthcare. Figure 1 shows the case selection criteria, inclusion and exclusion criteria applied in the study.
Case selection criteria
Specific ECtHR cases exemplify how the ECtHR navigates complex ethical dilemmas, balancing individual autonomy against public health considerations. These works provide critical insights into the Court’s evolving role as a mediator between biomedical progress and human rights protections. By employing these methods, the research achieves a holistic understanding of the ECtHR's role in harmonising biomedical progress with the protection of fundamental human rights. The combination of theoretical and practical legal approaches ensures that the findings are both analytically rigorous and relevant to current challenges in bioethics and human rights law.
Biomedical advancements have the potential to significantly enhance human health, extend life expectancy, and improve the quality of life, thereby supporting the fundamental human right to health. However, these advancements also pose unique challenges to human rights. Issues such as privacy and the confidentiality of genetic information, equitable access to advanced medical treatments, and the ethical implications of genetic modification and cloning raise critical concerns. The potential for discrimination based on genetic characteristics or health status necessitates robust legal and ethical frameworks to protect individuals' rights in the face of rapidly evolving biomedical technologies. Furthermore, the commercialisation of biomedical innovations can exacerbate inequalities, such as limiting access to life-saving treatments based on an individual's ability to pay. This underscores the need for policies that ensure fair and universal access to medical advancements, in line with the principle of equity in health care. However, while offering significant benefits, biomedical innovations pose potential risks to various human rights (Table 1).
Biomedicine is a relatively new phenomenon in the legal field. Nevertheless, there are already a number of international legal acts aimed at its regulation. In our opinion, the regulation of biomedical advances is a critical aspect of ensuring the balanced development of medical innovations and protection of fundamental human rights. This process requires a comprehensive approach, including the development and implementation of national and international regulations that meet ethical standards, protect patients' rights, and promote the safe use of biomedical technologies.
First, biomedical innovations can be defined as a component of the human right to life and health care. This follows from the fact that progress in the field of biomedicine is directly aimed at improving the health and well-being of people, expanding the possibilities of effective treatment of diseases, reducing mortality, and prolonging life expectancy. The development of new medical technologies plays a key role in the realisation of this right, providing individuals with the opportunity to lead a healthy lifestyle and receive highly effective medical care.
At this time, most of the issues related to modern advances in biomedicine have been resolved to some extent through the first and broadest-ever adoption of an act regulating social relations in this area, the Council of Europe Convention on Human Rights and Biomedicine [8]. This document transformed the accumulated experience and moral consideration of various advances in biomedicine into legal norms [6]. This Act establishes the basic principles of the relationship between biomedicine and human rights, in particular in areas such as genetics, organ transplantation, human research, and the right to privacy.
The Convention highlights the need to obtain free and informed consent from the individual before any medical intervention and guarantees the protection of personal data and confidentiality of medical information by giving the individual the right to access his or her own medical data. The document prohibits any form of discrimination on the basis of genetic inheritance and sets restrictions on the use of genetic interventions, in particular to change genetic characteristics that are transmitted to offspring, only for medical purposes and with appropriate safeguards. The Convention also provides for the protection of persons participating in research. It defines standards for the protection of persons participating in biomedical research, ensuring that their rights are adequately protected. In addition, the International Legal Act establishes an ethical and legal framework for organ and tissue transplantation, including a ban on the commercialisation of human body parts [8].
The Convention promotes international cooperation in the field of biomedicine by encouraging member states to share information, knowledge, and best practices. In addition, the principles and provisions of the Convention serve as a source of inspiration for national legislators, contributing to the development and improvement of national legislation in the field of biomedicine. The definition of clear ethical norms and legal limitations for biomedical interventions, particularly for genetics and transplantation, reflects the universal desire to preserve human dignity and respect for the individual in the context of the use of advanced technologies.
As noted by Krushelnytska [7] ensuring the rights of patients and participants in biomedical research is mainly viewed through the prism of respect for human biological materials, physical and mental health, and confidentiality of personal data. The Convention acts as a foundation that establishes unified standards in the field of bioethics, laying the groundwork for harmony between human rights and biomedicine [8]. This document builds international consensus on the need to limit scientific research and medical practices that may threaten fundamental human rights. Through the establishment of monitoring and mutual review mechanisms, the Convention ensures that States Parties maintain high standards of human rights protection in the field of biomedicine [8].
Furthermore, at the global level, there are three key international organisations; the UN, the WHO, and the UNESCO that focus on establishing rules for the use of human genetic information in the legal context. This division indicates the existence of an effective system of intergovernmental cooperation in this area. Although this cooperation usually does not lead to the creation of binding mechanisms that could directly influence the domestic policy and legislative activities of the participants, it leads to the creation of recommendatory acts, which promote standardisation and, accordingly, international legal regulation in this area [19].
Within the scope of international legal order, as well as national legal systems, law is founded on a normative system where two types of norms coexist: mandatory norms ("hard law") and non-mandatory norms ("soft law"). Bioethical norms are predominantly presented in the form of recommendatory standards (declarations, recommendations, ethical codes, statements, reports, etc.), which constitute acts of soft law [9].
An important legal act adopted at the universal level that is worth noting in the context of the research topic is the Universal Declaration on the Human Genome and Human Rights [20]. This Declaration recognises the human genome as a heritage of humanity and affirms the need to protect fundamental human rights in the context of genetic research and applications. The Declaration states that genetic research and its applications should respect human dignity, human rights, and fundamental freedoms, with particular emphasis on the non-discrimination based on genetic characteristics. The document outlines the right of individuals to confidentiality and the right to receive, or refuse to receive, information about their genetic status.
The Universal Declaration on the Human Genome and Human Rights [20] provides a framework for the development of national and international policies that ensure that genetic research is conducted with respect for ethical principles, the protection of human rights, and the promotion of universal values. The Declaration emphasises the need to protect individuals from any form of discrimination based on genetic information. It creates a just society where human rights are protected regardless of their genetic characteristics. The requirements for informed consent set out in the Declaration ensure that individuals have a full understanding of the potential risks and benefits before participating in genetic research or therapies. It is also worth noting that the Declaration highlights the importance of respect for human dignity in all aspects of genetic research, including the preservation of confidentiality, and personal information.
The next legal act is the Universal Declaration on Bioethics and Human Rights [21]. It establishes global principles of bioethics based on respect for human dignity, rights, and freedoms. This Declaration defines an ethical framework for scientific research and the application of technology, especially in areas related to biology and medicine, to protect the interests of individuals and communities. The document emphasises that all medical research and practice should be conducted with respect for human dignity and the protection of human rights. The Declaration emphasises the right of individuals to make decisions consistent with their own cultural, ethical, and religious beliefs. It also highlights the need to obtain informed consent from individuals before any medical intervention or research and provides for the protection of personal data. In addition, the Declaration calls for equitable access to health care and resources, as well as solidarity between peoples and generations.
Despite the extensive array of international guidelines, statements, and declarations on bioethics, the UNESCO Declaration stands out for its significant contribution to the field. It should be noted that this is the first international legal instrument, albeit non-binding, that addresses the relationship between human rights and bioethics in a comprehensive manner. Even with the limitations typical of such instruments, the achievement of consensus among virtually all states on this delicate issue is, in and of itself, a substantial accomplishment [12]. Thus, the Declaration establishes universal principles that aim to ensure that biomedical research and practice is conducted with respect for human rights, dignity, and freedoms. It forms an ethical framework for medical science and technology focused on the benefit of the individual. The regulation of biomedical advances requires not only a response to current challenges but also anticipation of future trends and potential risks associated with the development of medical technologies.
The ECtHR considers cases of violation of the European Convention on Human Rights [3]. In this context, many of the cases considered by the ECtHR are related to human rights violations in the context of biomedicine. The Court's practice is quite broad, so we will consider several key cases and provide the categories of rights that have been mostly violated.
The first case is Glass v. the United Kingdom [15]. This case addressed the issue of the right to respect for private and family life in the context of medical decisions, according to Article 8 of the European Convention on Human Rights [3]. The case concerned David Glass, a boy with serious medical conditions, including cerebral palsy. In March 1998, after another hospitalisation for respiratory problems, doctors decided to administer David a large dose of contraindicated drugs that they believed could lead to his death, with the aim of “reducing suffering”. It was noted in the medical record that no resuscitation would be performed. David's mother, Carol Glass, objected to this decision and argued that she had not consented to treatment that she believed was aimed at hastening her son's death. Glass's family filed a complaint with the ECtHR, alleging a violation of Article 8 of the European Convention on Human Rights - the right to respect for private and family life [3]. They argued that the actions of the medical staff, which were carried out without proper consent and against the mother's will, violated their rights.
In its judgment, the ECtHR recognised that the right to respect for private life of David and his mother had been violated. The Court emphasised that medical decisions, especially those involving potentially life-saving interventions, must be made with the full consent of the patient or, in the case of minors, their legal representatives. Interventions without such consent or the necessary legal basis may be recognised as a violation of the right to privacy [15]. This case had a significant impact on further understanding of the right of patients to informed consent and the importance of communication between medical staff and patients or their legal representatives, especially in critical and ethically sensitive situations. The judgment emphasises the need to ensure that medical decisions that may have irreversible consequences are based on clear consent and consider the patient's wishes and interests. The case also prompted medical institutions and health authorities in Council of Europe member states to review their policies and practices to ensure greater transparency and accountability in medical decision-making. The decision in Glass v. the United Kingdom case was an important precedent for the ECtHR, reinforcing the legal principles that protect patient autonomy and require medical professionals to obtain proper consent before conducting treatment, especially when it comes to life-saving or risky medical procedures [15].
There are also a number of recent decisions on Article 8 of the European Convention on Human Rights [3]. For example, the cases of Gauvin-Fournis v. France and Silliau v. France [22] related to the applicants born with the help of medical assistance at conception in the 1980s. They could not receive information about third-party donors involved in the process. A new legal system came into force on September 1, 2022. It was announced that persons born before would get access to information of their own ancestry, but it demands the donors’ consent. The Court concluded that the considered issue was the result of decisions made by the legislator. Each bioethical law was enacted after public consultations and debates to consider all viewpoints. The Court believed that the legislator considered the interests and rights involved in the decision carefully to annul donor anonymity. It was concluded that the issue of access to origin is still controversial. The Court considered that the legislator acted within its margin of appreciation. Thus, the state cannot be criticised for the slow tempo of the reform implementation or for its slow acceptance of it. The ECtHR, by a majority vote, found that there was no violation of Article 8 [22].
Moreover, the case of C v. Italy [16] concerned the refusal of the Italian authorities to recognise the legal relationship of paternity and maternity if a child was born due to surrogacy in Ukraine, which was confirmed by the birth certificate. The Court concluded that, according to Article 8 of the Convention, domestic law should provide for the recognition of the legal relationship between a child born as a result of surrogacy abroad and the intended father if he is the biological father. It was concluded that the legal relationship between the applicant, who was four years old, and the biological father had not been quickly established by the domestic courts making protection of the child’s interests impossible. Due to the situation, the child had a stateless status in Italy. On the Court decision, that the Italian authorities had failed to fulfil their positive obligation to ensure the applicant's right to respect her private life under the Convention [3]. Thus, the ECtHR found that there had been a violation of Article 8 of the European Convention on Human Rights regarding the establishment of legal relations between the applicant and her biological father [3]. At the same time, there was no violation of Article 8 regarding the establishment of legal relations between the applicant and her intended mother. Both of the cases under consideration concern Article 8 of the European Convention on Human Rights [3], but the court's decision differs based on different circumstances.
Regarding the violation of Article 3 of the European Convention on Human Rights [3], it is worth noting the case of G.M. and Others v. the Republic of Moldova [17]. It concerned the forced abortion and birth control measures against three women with intellectual disabilities who lived in a neuropsychiatric medical institution after they had been repeatedly raped by one of the chief doctors of the institution. The Court found that the authorities had failed to conduct an effective investigation into the allegations of ill-treatment, despite the fact that the investigation was reopened four times following their appeals. The investigation failed to consider their vulnerability as women with intellectual disabilities who had been sexually abused. As a result, it was concluded that effective protection against such invasive medical interventions realised without the patient's valid consent and was not provided by domestic criminal law.
Hence, the ECtHR unanimously concluded that a violation of Article 3 (prohibition of inhuman or degrading treatment) in both substantive and procedural terms took place. Specifically, regarding the lack of legal protection of the physical integrity of women with intellectual disabilities, the forced abortions of three applicants and the forced use of contraception in relation to the first applicant, as well as the obligation to conduct an effective investigation in relation to all three applicants [17]. This decision emphasises the importance of ensuring special protection of vulnerable groups, including persons with intellectual disabilities, in the context of medical interventions, and protection of their rights. In addition, the judgment draws attention to the seriousness of the issues associated with coercive medical interventions, such as abortion and contraception, especially when they are carried out without the patient's consent. This calls into question the ethics and legality of such actions in medical practice. Moreover, the Court pointed out the shortcomings in the investigation of crimes against vulnerable persons, especially when it comes to sexual violence and forced medical interventions. The decision emphasised the legal obligations of states to ensure adequate protection of human rights within the country, including the right to privacy and protection from inhuman or degrading treatment.
We would also like to draw attention to what we believe to be an important judgment concerning the issue of end of life. In the case of Mortier v. Belgium [23], the applicant complained of a violation of two articles, namely: Articles 8 (right to respect for private and family life) and 2 (right to life). This case concerned the death of the applicant's mother by euthanasia without notifying the applicant or his sister. The applicant's mother did not want to inform her children about her request for euthanasia, despite repeated recommendations from doctors. The applicant, relying on Article 2 (right to life) argued that the state had failed to fulfil its obligations to protect his mother's life, as the legal euthanasia procedure had allegedly not been followed in her case. He also complained about the lack of in-depth and effective investigation into the violations he alleged. Referring to Article 8 (the right to respect for private and family life), Mr. Mortier argued that the state had violated this Article by failing to ensure effective protection of his mother's right to life [23].
The court came to quite different conclusions. Thus, it noted that there had been no violation of Article 8 of the Convention and also pointed out that there had been no violation of Article 2, given the legal framework governing acts and procedures of pre-euthanasia and the conditions in which the act of euthanasia was carried out in a particular case. However, Mr. Mortier continued to argue for a violation of Article 2 of the Convention, in view of the procedure for reviewing the decision on euthanasia [23]. This case highlights the difficulty of striking a balance between the right to life, as required by Article 2 of the European Convention on Human Rights, and the right to autonomy, including the ability to decide on euthanasia [3]. The Court examines these issues through the prism of a legal framework that must ensure adequate protection of both rights. The Court emphasised the importance of the procedural aspect of the right to life, pointing out the need for effective investigation of cases where this right may be violated. An independent and effective investigation is critical to ensuring justice and preventing impunity. The case also highlights the importance of medical confidentiality and ethical guidelines in cases where a person chooses not to inform relatives of his or her decision to be euthanised. This raises discussions about the limits of medical confidentiality and the duties of doctors to the patient and his or her family.
Finally, the case of Dolenc v. Slovenia [24] concerned a violation of Article 6 (right to a fair trial) of the European Convention on Human Rights [3]. The case concerned an Israeli citizen who was left paralysed after an operation performed by the applicant, a well-known neurosurgeon, in a Ljubljana hospital, and subsequent trials in both Israel and Slovenia. Referring to Article 6 (the right to a fair trial), Mr. Dolenc argued that the Slovenian courts should refuse to recognise the Israeli judgments, which awarded the applicant's former patient more than EUR 2 million, as they were made as a result of an unfair trial. The court found that before recognising the Israeli judgments, the Slovenian courts had not properly verified that the trial in Israel was fair. In particular, there were problems with the collection of evidence. The court in Israel did not listen to important witnesses such as hospital staff and an expert in Slovenian law, and excluded their statements from the case file. In this context, the court found a violation of Article 6 (right to a fair trial) of the European Convention on Human Rights [3].
Table 2 summarises the key facts, violations, and outcomes of each case that was analysed in the research. The table is presented by the relevant articles of the European Convention on Human Rights.
Thus, if we analyse the large number of cases that have been considered by the ECtHR recently, we can distinguish the following articles of the European Convention on Human Rights, the violation of which the applicants complained about most often: Article 8 (right to respect for private life); Article 2 (right to life); Article 6 (right to a fair trial); Article 14 (prohibition of discrimination); Article 3 (prohibition of torture) [3]. Most of the cases reviewed are rather new (2022-2023), which indicates that the relevance of human rights violations related to biomedicine is not diminishing and is taking on new forms. This indicates the need to increase the protection of inalienable human rights in the implementation of medical measures. In this context, the ECtHR is also important, as it develops case law that must be followed by all Council of Europe member states and influences their legislation.
Even though the issue of human rights observance in the context of biomedicine is quite researched, the analysis of practice has shown many recent ECtHR judgments on this issue. The importance of the ECtHR lies in its ability to consider complaints from individuals, which enables victims of human rights violations in the field of biomedicine to seek international protection. We can identify five main areas in which the Court's law-making plays an important role:
Thus, the cases considered by the ECtHR in the context of biomedicine not only contribute to the development of legal norms and practices but also play a key role in shaping ethical standards in the field of healthcare. Through these judgments, the Council of Europe member states receive important guidance on how to strike a balance between scientific progress and fundamental human rights, while considering the ethical, social, and legal challenges facing modern society.
Therefore, with the rapid development of biomedicine, which offers significant opportunities for improving health, new challenges arise in the areas of privacy, access to healthcare, genetic integrity, etc. The development of biomedical innovations poses challenges to society that require a careful balancing act between progress in healthcare and the protection of constitutional human rights.
The ECtHR plays a crucial role in shaping the case law that protects human rights in the context of medical and biological innovations, setting important precedents for member states. Having analysed the most recent ECtHR judgments, the author has identified several articles of the European Convention on Human Rights, the violation of which has been the subject of complaints most often: Article 8 (right to respect for private life), Article 2 (right to life), Article 6 (right to a fair trial), Article 14 (prohibition of discrimination) and Article 3 (prohibition of torture) [3]. The timing of these cases (2022-2023) highlights the ongoing relevance and evolution of human rights violations in the field of biomedicine, pointing to the need to strengthen the protection of fundamental rights in the medical context. The analysis of the ECtHR case law shows that biomedical innovations and interventions may violate inalienable human rights. As such, the ECtHR case law emphasises the need to ensure transparency, informed consent, and confidentiality in the medical field, which are key elements of human rights protection.
In addition, the cases considered by the ECtHR indicate the need for further development and improvement of national legislation to address modern biomedical challenges and ensure adequate protection of human rights. The active role of the ECHR in addressing biomedicine-related issues not only contributes to the protection of individual rights but also stimulates global dialogue and cooperation in the field of bioethics, which contributes to a more humane approach to medical innovations at the international level.
The ECtHR’s law-making is of key importance in some key areas. For example, it serves as case law, setting guidelines for national courts, thereby contributing to the consistency of human rights protection. Biomedicine related judgments assert the need to protect human rights in the medical context, in particular the right to privacy, informed consent, and access to medical care. They also encourage states to reform their national legislation to comply with international standards. In addition, important areas include the protection of the rights of vulnerable groups and the need for international cooperation to address transnational issues in the field of biomedicine.
1. Kozodayev CP, Bysaha YM, Bielov DM, Hromovchuk MV. Protection of Constitutional Human Rights and Freedoms in the Process of Biomedical Research. Uzhhorod: Helvetica; 2018
2. Hendel NV. Protection of Biomedical Human Rights in Practice of the European Court of Human Rights. Legal Support for the Effective Enforcement of Judgments and Application of the European Court of Human Rights Case Law. 2013;2:414–21.
3. European Convention on Human Rights. 1950. Available from: https://www.echr.coe.int/documents/d/echr/convention_ENG (assessed 29 March 2024).
4. Tarasevych T, Yuzko T, Hrabovska O, Romanova O, Lisova K. Peculiarities of Consideration of Cases in the ECtHR regarding the Protection of Constitutional Human Rights Related to the Fourth Generation of Somatic Rights. Jurid Tribune. 2023;13(4):644–67. doi:10.24818/TBJ/2023/13/4.09.
5. Van Drooghenbroeck S, Rizcallah C. The ECHR and the Essence of Fundamental Rights: Searching for Sugar in Hot Milk? Ger Law J. 2019;20:904–23.
6. Mikhalov VO. Medical and Genetic Human Rights (on the Problem of Their Regulation). Legal Life Mod Ukr. 2020;1:337–9.
7. Krushelnytska H. Features of Legal Regulation of Biomedical Technologies in the Nordic Countries. Legal Bulletin. 2022;5:206–18.
8. Convention on Human Rights and Biomedicine. 1997. Available from: https://www.coe.int/en/web/impact-convention-human-rights/convention-on-human-rights-and-biomedicine# (assessed 29 March 2024).
9. Ostrovska B. Development of Bioethics in International Law as a Global Platform for Solving Human Rights Problems. Philos Methodol Probl Law. 2019;17(1):25–31.
10. Prainsack D, Buyx A. Solidarity in Biomedicine and Beyond. Cambridge: Cambridge University Press; 2017. doi:10.1017/9781139696593
11. Rendtorff JD, Kemp P. Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. Biolaw Policy 21st Century Build Answers New Questions. 2019;78:33–40.
12. Andorno R. Global Bioethics at UNESCO: In Defence of the Universal Declaration on Bioethics and Human Rights. J Med Ethics. 2007;33(3):150–4.
13. Gostin LO, Wiley LF. Public Health Law: Power, Duty, Restraint. Berkley: University of California Press; 2016.
14. Yamnenko TM, Litvinova IF. Protection of Personal Data in the Healthcare Sector (Criminal Law Aspects). Legal Bull. 2019;1(50):185–91.
15. Case Glass v. United Kingdom. Application no. 61827/00. 2004. Available from: https://hudoc.echr.coe.int/Eng?i=001-61663 (assessed 29 March 2024).
16. Case C v. Italy. Application no. 47196/21. 2023. Available from: https://hudoc.echr.coe.int/eng?i=001-226391 (assessed 29 March 2024).
17. Case G.M. and Others v. the Republic of Moldova. Application no 44394/15. 2023. Available from: https://hudoc.echr.coe.int/fre?i=001-220954 (assessed 29 March 2024).
18. Senyuta IY. Human Right to Health Care and Freedom. Committee on Medical and Pharmaceutical Law and Bioethics. 2019. Available from: https://medcom.unba.org.ua/publications/4397-pravo-lyudini-na-medichnu-dopomogu-ta-svoboda.html (assessed 29 March 2024).
19. Zhornytskyi VM. UN System Institutional Aspect of International Legal Regulation of Human Genetic Data Handling. Comp Anal Law. 2020;2:256–9.
20. Universal Declaration on the Human Genome and Human Rights. 1997. General Conference of the United Nations Educational, Scientific and Cultural Organization at its twenty-ninth session. Available from: https://www.ohchr.org/en/instruments-mechanisms/instruments/universal-declaration-human-genome-and-human-rights (assessed 29 March 2024).
21. Universal Declaration on Bioethics and Human Rights. 2005. Available from: https://www.unesco.org/en/legal-affairs/universal-declaration-bioethics-and-human-rights?hub=66535 (assessed 29 March 2024).
22. Case Gauvin-Fournis and Silliau v. France. Applications no. 21424/16 and 45728/17. 2023. Available from: https://hudoc.echr.coe.int/fre?i=002-14177 (assessed 29 March 2024).
23. Case Mortier c. Belgique. Application no 78017/17. 2022. Available from: https://hudoc.echr.coe.int/fre?i=001-219559 (assessed 29 March 2024).
24. Case Dolenc v. Slovenia. Application no 20256/20. 2023. Available from: https://hudoc.echr.coe.int/eng/?i=001-219946 (assessed 29 March 2024).