Watching Brief
| Date of first report of the outbreak | 20 December 2023. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Disease or outbreak | Western Equine Encephalitis (WEE). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin (country, city, region) | Western Equine Encephalitis virus (WEEV) is a new-world alphavirus (family Togaviridae), discovered in 1930 when it was first isolated from a horse brain in California, the United States (US), during an encephalomyelitis outbreak among horses in the San Joaquin valley [1,2]. The virus was later isolated from a human in 1938 at the University of California, from the brain of a child who had deceased [3]. WEEV circulates mainly in the western regions of the US and Canada, and the southern cone of South America, encompassing Argentina, Chile and Uruguay [4]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Suspected Source | Human WEE cases are often associated with equine cases as mosquitoes transmitting infection between horses may also bite humans [1]. Since late November 2023, both Argentina and Uruguay have experienced a large number of equine outbreaks[ 4 ]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Date of outbreak beginning | On 25 November 2023, Argentinian officials at the National Food Safety and Quality Service (SENASA) received reports of positive results for an unspecified alphavirus from equine samples in the provinces of Corrientes and Santa Fe [ 5 ]. Equine infection with WEEV was confirmed on 27 November 2023[ 5 ]. Additional equine cases were soon identified in six other provinces, prompting declaration of a nationwide health emergency, triggering containment measures such as restricting the movements of horses from affected provinces and requiring immediate notification of any suspected neurological diseases in equines[ 5 , 6 ]. The first human patient in Argentina developed symptoms on 19 November 2023 [ 7 ]. In Uruguay, an equine WEE case was confirmed on 5 December 2023, in Salto municipality [ 5 ] and the first human patient in Uruguay developed symptoms in early January 2024 [ 8 ]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Date outbreak declared over | In Argentina, the outbreak is ongoing as of 16 July 2024[ 9 ]. In Uruguay, as of 30 April 2024, officials reported the outbreak over, at least in equines, as no additional equine cases had been reported for a period of 12 weeks since the last case[ 10 ]. No further human cases have been reported since March. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected countries & regions | In Argentina, confirmed human cases have been reported from Buenos Aires, Santa Fe, Entre Ríos, Córdoba, Río Negro, La Pampa, Buenos Aires City, and Santiago del Estero [ 9 ] (Figure 1). Suspected and probable cases have also been reported in Mendoza, San Juan, San Luis, Chaco, Corrientes, Catamarca, Jujuy and La Rioja[ 9 ]. In Uruguay, confirmed human cases occurred in San José, Maldonado, and Montevideo [ 11 ] (Figure 2). Suspected cases have also been reported in Canelones, Salto, Cerro Largo, Colonia, Durazno, Lavalleja, Río Negro, Rivera, Soriano, Artigas, Paysandú, and Rocha[ 11 ]. In both countries, confirmed human cases are identified based on laboratory diagnosis, and suspected cases are defined as having unexplained fever of sudden onset with headache or myalgia without upper airway involvement, and with unexplained neurological manifestations[12-14]. Confirmed equine cases in Argentina require a positive laboratory test, while in Uruguay, confirmed cases are reported as dead horses with a positive test[ 15 ]. In both countries, suspected cases are those with clinical symptoms[ 15 ]. Brazil has also officially confirmed an equine case, on 26 January, in Barra do Quaraí, Rio Grande do Sul, though no human cases have yet been reported [ 11 ]. In late November, following the cases in Argentina, the Brazilian government issued an information notice regarding WEE, stressing that any suspected cases of neurological diseases in horses must be reported for official investigations[ 16 ], however, such protocols have been in place since 2003 with the establishment of the National West Nile Fever Surveillance System [ 17 ]. However, a recent study has reported on the detection of WEEV RNA in the brains of three horses who died between late December 2023 and late January 2024 in Rio Grando do Sul, who were unvaccinated and had exhibited neurological signs[ 18 ]. . |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
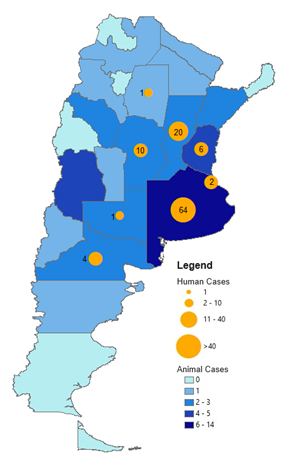
| Number of cases (specify at what date if ongoing) | As of 16 July 2024, 47 confirmed and 1,482 suspected equine cases were reported across 18 provinces in Argentina, mostly in Buenos Aires[9 ]. As of 16 July 2024, there have been 108 confirmed human cases in Argentina and 12 deaths [ 9 ] (Figure 1).
Figure 1
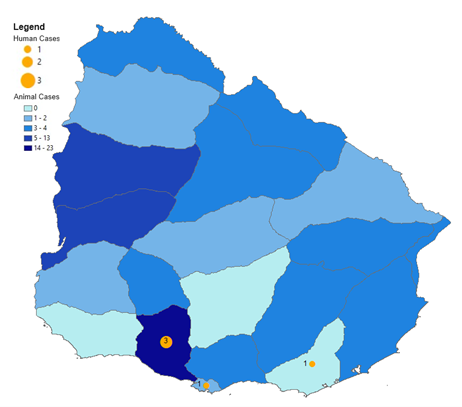
Confirmed human and equine cases of Western Equine Encephalitis reported since outbreak onset until 16 July 2024 in Argentina[ 15 ]. Blue shaded regions indicate provinces in which equine cases have been reported. Orange circles indicate human cases. Data sourced from: Encefalitis Equina del Oeste en la Región de las Américas [Western equine encephalitis in the Region of the Americas]Pan American Health Organization. Accessed at: https://shiny.paho-phe.org/encephalitis/ In Uruguay, there were 80 confirmed and 940 suspected equine cases, mostly in San José, while there have been five confirmed human cases, with no deaths [ 15 ] (Figure 2).
Figure 2
Confirmed human and equine cases of Western Equine Encephalitis reported since outbreak onset until 16 July 2024 in Uruguay[ 15 ]. Blue shaded regions indicate departments in which equine cases have been reported. Orange circles indicate human cases. Data sourced from: Encefalitis Equina del Oeste en la Región de las Américas [Western equine encephalitis in the Region of the Americas]. Pan American Health Organization. Accessed at: https://shiny.paho-phe.org/encephalitis/ |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical features | In humans, infections are usually asymptomatic or mild with symptoms that generally present within 5-15 days, including fever, headache, chills, nausea and vomiting, lack of appetite, malaise and myalgias[ 19 ]. However, with a sufficiently high viral load, WEEV may enter the central nervous system (CNS), causing cerebral and meningeal inflammation[19]. This may cause stiff neck, photophobia, confusion, seizures, and reduced consciousness or coma[ 20 ]. WEE is typically more severe in infants, young children, and the elderly[ 21 ]. In Argentina, clinical information was available for 106 patients, with the most common signs being: fever (n=82 [77.4%]), headache (76 [71.7%]), confusion (63 [59.4%]), drowsiness (37 [34.9%]), other neurological manifestations (33 [31.1%]), vomiting (31 [29.2%]), myalgia (24 [22.6%]), tremors (17 [16.0%]), and despondency (13 [12.3%])[15]. In Uruguay, it has been reported that four of five (80.0%) cases developed encephalitis, and one (20.0%) case had mild disease[ 22 ]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mode of transmission (dominant mode and other documented modes) | WEEV is transmitted primarily by the bite of an infected mosquito. Most non-human cases (96.9%) occur in horses[ 23 ]. Human WEE cases are rare and usually occur following equine cases, as individuals working outdoors are at greater risk of contact with mosquitoes([ 1 , 7 ]. In both Argentina and Uruguay, human cases were reported alongside equine cases, and most deaths in Argentina had outdoor exposure [9] (Table 1), and most cases in Uruguay had contact with horses [ 22 ] (Table 2). In the Northern hemisphere, the Culex tarsalis and Aedes melanimon mosquitoes are important vectors, and passerine birds are the primary reservoir host[ 24 ]. In South America, studies indicate Aedes albifasciatus as a potential vector[ 25 ], with various birds and mammals acting as reservoir or amplification hosts[ 21 , 24 ]. Horses and humans are generally considered incidental dead-end hosts that cannot transmit WEE to each other[ 24 ], though some equids may develop viral loads capable of infecting mosquitoes, thus their role as potential amplification hosts requires further investigation[ 21 ]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Demographics of cases | Available demographic characteristics for confirmed human cases and deaths in Argentina and confirmed cases in Uruguay are presented in Tables 1 and 2, respectively. Most cases are male, and most cases are aged between 40 to 79 years. This may be due to differences in symptomology or testing patterns (for example, due to differences in occupational exposure risk) by sex or age, and further analysis is needed to elucidate any associations between these factors and disease outcomes. Table 1: Characteristics of confirmed Western Equine Encephalitis cases and fatalities in Argentina(9).
*Percentage values may not total 100 due to rounding. Data sourced from: Boletín Epidemiológico Nacional: Semana Epidemiológica 27 [National epidemiological Bulletin: epidemiological week 27], Ministry of Health of Argentina, 2024. Table 2. Characteristics of confirmed Western Equine Encephalitis cases in Uruguay[ 22 ].
Data sourced from: Ministerio de Salud Pública. Reporte Semanal 26/03/2024: Vigilancia de Encefalitis Equina del Oeste-encefalitis/meningitis de probable etiología viral [Weekly Report 26/03/2024: Surveillance of Western Equine Encephalitis-encephalitis/meningitis of probable viral etiology]. Uruguay, 2024. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Case fatality rate | The WEE case-fatality rate (CFR) in humans is between 3-7%[26]. Deaths occur mostly in older patients[19]. In encephalitis cases the CFR is ~5-15%[27]. The CFRs for the current outbreaks are 11.1% (12/108) and 0% (0/5) in Argentina and Uruguay, respectively[ 9 , 11 ].. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Complications | Neuroinvasive disease most commonly affects infants, young children, and older patients[ 19 ]. Up to 30% of patients with neurological disease will experience significant persistent neurological problems, with infants and very young children at highest risk of permanent disability[ 19 , 26 , 28 ]. Neurological sequelae may include cognitive or behavioural disorders, seizures, spasticity, depression, and anxiety[ 19 , 28 ]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Available prevention | WEE prevention involves reducing contact with mosquitoes[ 5 ]. This may include environmental measures (e.g. filling in or draining areas that may serve as breeding sites, eliminating weeds, and using mosquito nets in stables, etc.), and insecticide spraying in areas with high mosquito populations[ 5 ]. Personal protective measures include using mosquito nets, long-sleeved clothing and repellents, and avoiding outdoor activities during dusk and dawn when mosquitoes are most active[ 8 ]. Both Argentina and Uruguay have recommended that individuals implement personal prevention measures[ 29 , 30 ]. Disease surveillance can also help prevent outbreaks by detecting high WEEV circulation. In both Argentina and Uruguay, suspected cases in horses or humans must be notified[ 7 , 31 ]. Other surveillance methods include using sentinel birds to assess seroconversion and trapping adult female mosquitoes for testing[ 1 ]. Multivalent formalin-inactivated vaccines for horses are available and efficacious in protecting against clinical disease after two initial doses, with annual boosters recommended[ 1 , 8 , 32 ]. High vaccination coverage should be maintained for horses in at-risk areas[ 8 ]. In Argentina, mandatory vaccination was introduced for all horses that were at least two-months-old on 24 January 2024[ 33 ], while Uruguay was able to procure a significant stock of WEE vaccines and vaccinated a “large population” of horses[ 12 ]. Uruguay also implemented movement restrictions on animals from affected areas[34]. Research into developing human alphavirus vaccines have been ongoing for decades[ 35 ]. The US army has administered an experimental inactivated WEEV vaccine under Investigational New Drug protocols, and found it to be immunogenic, safe, and well-tolerated[ 35 ]. However, due to manufacturing constraints, and a focus instead on next-generation trivalent vaccines (for WEEV, Eastern Equine Encephalitis virus and Venezuelan Equine Encephalitis virus), there is currently no licensed WEEV vaccine available for humans[ 35 ]. Although there have been considerable advances in WEEV vaccine research, current challenges include safety concerns and lack of protection against heterologous challenge[ 28 ]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Available treatment | As there is no cure for WEE, treatment is largely supportive and aimed at managing complications, using antipyretics, analgesics, and anticonvulsants[ 19 ,36 ]. However, several studies have indicated potentially promising therapeutic candidates. Nucleoside analogues, such as ribavirin, have demonstrated some inhibitory activity in vitro, however, studies have yet to show effectiveness in humans[1]. Studies in mice have also found an adenovirus-mediated expression of interferon alpha that provided pre- and post- exposure protection, cationic liposome-DNA complexes that stimulate the innate host immune response, and human-like monoclonal antibodies that protect against WEEV aerosol exposure[ 37 - 39 ]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Comparison with past outbreaks | Human WEE cases have previously been reported in Argentina in 1982-83, during a severe epizootic in horses that began in Santa Fe and extended south to Viedma, Rio Negro province[40], and in 1996 when an isolated case was confirmed but no equine cases were identified[7]. In Uruguay, a previous human case was reported in Montevideo, Uruguay in April 2009. A 14-year-old boy was hospitalised following four days of fever, asthenia, and headaches, however, his condition deteriorated and he died three days later[41]. Prior to this case, WEEV had not been documented in Uruguay since the early 1970s, when several equine outbreaks occurred and a serologic study found WEEV antibodies in children[41]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unusual features | Overall, global reports of WEE human infections and enzootic WEEV circulation have decreased substantially in recent decades, possibly due to ecologic factors, such as the declining use of equids in agriculture, and declining and/or changing vector and host populations, among others[21 ,42]. Therefore, the large number of cases in Argentina and Uruguay are unusual, as the previous cases in Argentina and Uruguay occurred 15 and 28 years ago, respectively, and both were isolated cases. However, cases could have occurred in the meantime and were simply not diagnosed or reported[41]. Recent El Niño events, causing increased rainfall and warmer temperatures along the west coast of South America[43], could also be partly driving the current outbreak. Despite being neighbouring countries, Uruguay has reported substantially fewer equine and human WEE cases than Argentina. This may be due to officials being forewarned by the outbreak in Argentina and thus better prepared to initiate surveillance, prevention, and response measures, whereas, in Argentina, suspected equine WEE cases were already reported from multiple provinces at outbreak onset, indicating wide undetected spread of the disease by the time the first notification was made. In comparison, officials in Uruguay issued a statement on 27 November, urging the notification of neurological symptoms in equines[5]. As Argentina has eight times more land area dedicated to agriculture compared to Uruguay and approximately six times as many horses (being one of the largest South American producers of horsemeat), exposure risk to WEEV vectors may be greater in Argentina[ 44 -47]. Proximity to farmland and areas with large horse populations, as well as human population density, may also influence exposure risk. For example, most equine and human WEE cases in Argentina have occurred in the north-east of the country, where both equine density and human population density are greatest[ 48 ,49]. However, further investigation would be required to confirm any association between these factors. The WEE case numbers in Argentina may also be exacerbated by the country’s severe ongoing dengue outbreak, placing great strain on public health systems and resources with over half a million cases reported in the first 23 weeks of 2024[50]. Lastly, although WEE is notifiable in both countries, health-seeking, testing and reporting behaviours may differ within and between the countries, potentially affecting case numbers. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Critical analysis | The current South American WEE outbreak demonstrates the significant morbidity that WEEV may present. Other arboviruses, such as dengue and chikungunya, are also increasing in South America, while others are re-emerging, such as yellow fever in Venezuela[51 -53]. Thus, further efforts are needed, which may benefit from a One Health approach, to help alleviate the increasing pressures caused by arboviral diseases on health systems in South America. Arboviral disease burdens have increased globally in recent decades[54]. The current WEE outbreak and increasing risk of human exposure to WEEV vectors may be due to various seasonal, climatic, anthropological, and socio-economic factors. El Niño events, which are forecasted to persist until May 2024 and are known to be associated with South American dengue outbreaks[ 55 ,56], as well as climate change, favour the spread of mosquito vectors by increasing rainfall and temperatures[11]. The current outbreak also coincides with summer, when vectors may prefer feeding on mammals[11]. Other environmental changes (e.g. deforestation, increased urbanisation and transportation), also facilitate the spread of mosquitoes[ 11 ,57]. The outbreaks in Argentina and Uruguay support the importance of such environmental factors, as most deaths from Argentina and most cases from Uruguay were associated with, respectively, spending time in a rural/semi-rural area and contact with horses[ 9 ,22]. Socioeconomic factors may also increase the WEE disease burden (e.g. due to lack of affordable healthcare; or use of water storage containers due to intermittent access to water[58], etc.). Thus, addressing arboviral diseases requires a holistic approach that considers the diverse factors directly and indirectly impacting vector-host interactions. There is also an urgent need for additional prevention measures using new and developing technologies. Outdoor insecticide-laced sugar baits laced and indoor passive emanator spatial repellents have shown promising results in protecting against mosquitos[ 59 ,60]. Other measures include new types of insecticide-treated nets, genetic modification, and the use of endectocidal drugs which, when administered to humans or animals, cause blood-feeding arthropods to die after feeding[ 59 ,61]. Ivermectin has demonstrated effectiveness against mosquitoes and has a well-established safety profile[61]. However, insufficient evidence exists to demonstrate the impact of human endectocidal drugs targeting mosquitos or to inform official guidelines on ivermectin endectocidal use[61]. The first large-scale clinical trial is underway in Mozambique and Kenya, with completion estimated in October 2024[62]. Across the Americas, vector control is suboptimal, likely due to factors including inadequate waste control, underfunding, lack of training and expertise, and diversion of resources during the COVID-19 pandemic[11]. Argentina is also currently facing a repellent shortage[63], which may hinder prevention efforts and lead to further cases of WEE or other arboviruses. Considering the increasing arboviral disease trends, urgent action is needed to develop and inform the use of therapeutic and non-therapeutic measures for vector control and distribute them efficiently to large populations. Efforts to improve WEE disease surveillance are also needed, for early detection of outbreaks and changes in virus pathogenicity or transmission. Although a network of 40 diagnostic laboratories, the Arbovirus Diagnosis Laboratory Network of the Americas (RELDA), was established in 2008, laboratory guidelines for detecting and diagnosing WEE human infection were only released in December 2023[ 64 ], raising concerns that potential WEE cases during this outbreak and in previous years were missed. Underreporting is also more likely as most countries in the Americas do not have a mandatory notification requirement for WEE, and surveillance may be limited by concurrent outbreaks of other diseases, including dengue, of which cases in 2024 have reached record-breaking levels[ 61 ,65]. Given the known association between equine and human WEE cases, which is seen in the current outbreaks, sentinel surveillance in horses may be considered, however, in Argentina, the first human case occurred prior to notification of the equine cases, and vaccination may limit their effectiveness as sentinels. However, the outbreaks in Argentina and Uruguay underscore the importance of both human and animal health surveillance and reporting in addressing the overall WEE disease burden. Surveillance is also important for investigating spatiotemporal phylogenetic differences in WEEV strains, as an understanding of viral characteristics such as transmission or virulence may help with outbreak control. The South American strain has historically been considered less virulent than the North American strain[66]. However, the recent study that detected WEEV RNA in the three horses from Brazil found that the cases had a novel WEEV lineage, closely related to the CB87 strain reported in Argentina in the 1960s, which may be more virulent than currently circulating North American strains[18]. These findings suggest that WEEV has been evolving and circulating in South America for decades, and that cases have likely been unreported[18]. Thus, surveillance efforts are vital for identifying early outbreaks signals or changes in virulence or transmission which may have significant implications for limiting disease burden. Although the current outbreak may be fuelled partly due to temporary climatic phenomena, arboviral disease burdens have been increasing globally for decades. Though the current WEE outbreak in South America may be unusual, it may not be the last. Current prevention and response measures are insufficient; public health policies must consider the various factors contributing to the continuing increase in arboviral diseases and support the development and large-scale implementation of new and improved vector control methods and improvement of disease surveillance. Given the complex nature of arboviral disease outbreaks and epidemiology, long-term sustainable solutions must involve a more proactive, unified and integrated approach that improves the health of humans, animals and the environment in tandem. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key questions |
• What would be the characteristics of a successful WEEV vaccine and what vaccination strategy should be implemented? • What can neighbouring countries do to prevent further WEE spread in South America? • How might health policies and resources be structured to encourage development, upscaling and implementation of new WEEV prevention and treatment measures? • What more can be done to help prioritise arboviral disease prevention and treatment in research, particularly as cases continue to increase globally? • How can countries safeguard against the changing climatic factors that favour WEEV transmission? • What factors are contributing to the timing and large scale of the current outbreak, given that global reports of WEE cases and enzootic circulation have been declining in recent decades? • How can countries improve surveillance measures in humans, sentinel animals and vectors, and ensure strengthened reporting of suspected cases? • What would a One Health approach to addressing WEE and other arboviral disease burdens look like and how might it be implemented within and across countries? |