Dengue is an acute viral infection caused by the dengue virus, belonging to the family Flaviviridae, and is spread by the bite of an infected Aedes aegypti or Aedes albopictus mosquito. Due to its associated symptoms of myalgia and arthralgia, dengue is also termed “breakbone fever” [1]. Dengue fever and its complications have risen continuously over the past 40 years [2]. Annually, 20,000 deaths are recorded globally due to dengue, despite 99% of deaths being avoidable [3]. The clinical spectrum of the disease varies from asymptomatic to severe and fatal forms. Dengue has an incubation period of 4-7 days and may present with an acute high-grade fever, blurred vision, subconjunctival hemorrhage along with retro-orbital pain and arthralgia. Thrombocytopenia occurs in about 70% of dengue patients [3]. In severe cases, it may lead to bleeding under the skin and mucosal surfaces, gastrointestinal bleeding, severe liver pathology, encephalitis, and renal dysfunction [4]. If not managed appropriately, this might also lead to dengue shock syndrome (DSS) [5,6]. Currently, fluid therapy remains the mainstay of treatment for severe dengue [7,8]. In a cohort study conducted in Nicaragua by Gordon et al., secondary infections occur in 59.6 to 115.2 infections per 1,000 persons annually, with the highest incidence in children aged below 5 years [9]. The present research mainly focusses on clinical presentations and the complications associated with dengue fever.
The present observational study was conducted at three tertiary care hospitals in and around Mangalore. Prior permission was obtained from the Medical Superintendent and District Medical Officer.
The study was conducted in accordance with the ethical principles following approval from the Scientific Review and Ethical Committee (IEC KMC MLR 04-16/85). All reported dengue cases in the hospitals were admitted from 2015 to 2019. All the cases were confirmed with the following laboratory investigations: immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA), nonstructural protein 1 (NS1) Antigen ELISA, and reverse transcription polymerase chain reaction (RT-PCR) [10]. Patients with other viral or bacterial infections after a routine lab test and those who refused to participate in the survey were excluded from the study.
Data obtained was recorded in Microsoft Excel and analysis was done using SPSS version 16.0. Results were presented using tables and figures.
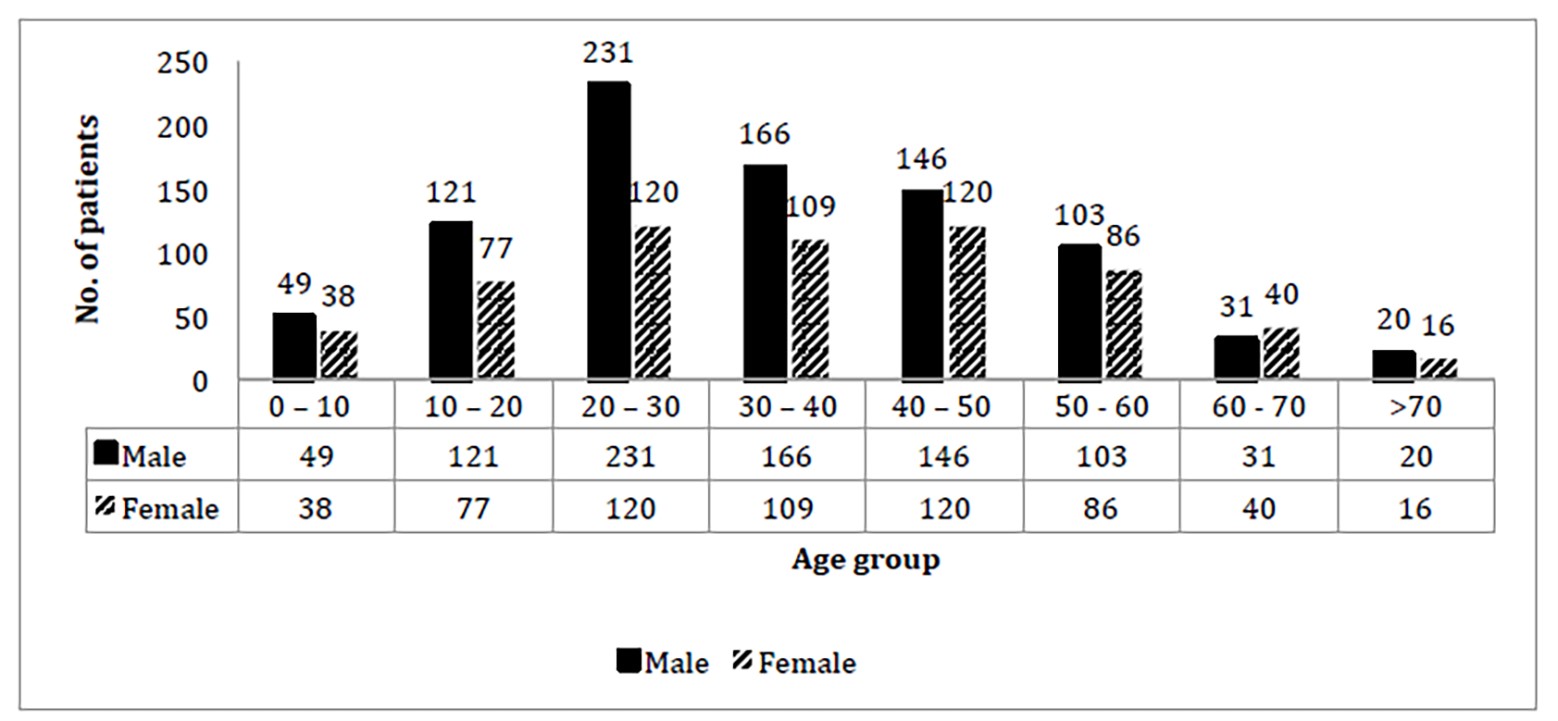
The total patients of 1,474 were enrolled. Among them, 869 (58.9%) were males and 606 (41.4%) were females. Among them, 942 (63.9%) were married and 532 (36.1%) were unmarried. Most of the patients were manual laborers. The age range was 1 year to 85 years. There were 351 patients belonging to the 20-30 years age group, of which 231 were males and 120 were females (Figure 1). Most patients were from Mangalore. The 731 patients from outside of Mangalore were from Belathangady, Sullia, Puttur, Bantwal, Udupi, Bangalore, Moodbidri, Davangere, Karwar, Coorg, Belgaum, Uttara kannada, Shimoga, Chikkamagalur, Hassan, Haveri, Bagalkote, Gadag, Koppal, and Chitra Durga. Nine patients were from other states, including West Bengal, Madhya Pradesh, Pune, Uttar Pradesh, Rajasthan, Assam, Mumbai, and Andhra Pradesh. Fever with headache was the most common symptom, while hypotension along with abdominal pain were the most frequent signs (Table 1). Forty-eight cases were admitted to the intensive care unit due to hematological complications. Twelve patients had macrophage activation syndrome. Pancytopenia was seen in five patients. Disseminated intravascular coagulation (DIC) and deep venous thrombosis (DVT) were complications in one of the patients (Table 2). Laboratory investigations were categorized into high, normal and low levels. Electrolytic imbalances were observed, particularly hyponatremia (35.3%) and low bicarbonate levels (78.8%) (Table 3), however, there were no significant statistical correlations between either hyponatremia (χ2{1,N=1474}=2.0047, p=0.156815; p >0.05) nor low bicarbonate levels (χ2{1,N=1474}=1.4764, p=0.224338; p >0.05), with respect to the complications of dengue fever. Chest X-ray was done in 324 patients, out of which 9.25% had pleural effusion. Pneumothorax was seen in three patients. Ultrasonography (USG) (Table 4) revealed changes in liver parenchyma of 106 patients and hepatomegaly in 89 out of 358 patients who underwent the scan. Overall, 132 scans showed gallbladder wall edema while 127 patients had ascites and 82 patients had splenomegaly. Gallstones were found in 19 patients and portal hypertension in nine patients. Among the 19 patients subjected to brain scans, cerebral edema was observed in three patients, and ischemic changes were seen in two patients. Post-viral demyelination was observed in one patient. Electrocardiography (ECG) and echocardiography (Table 4) revealed sinus tachycardia in 21 patients, ST elevation in 13 and T-wave abnormalities. These changes are suggestive of myocarditis. Myocardial infarction was seen in ten patients. Pericarditis and pericardial effusion were each seen in one patient. Left ventricular hypertrophy was seen in 27 patients.
Cross-tabulation of Dengue Fever with Age Group and Gender
Table 1
Symptoms and Signs of Patients with Dengue Fever
| Symptoms (Seen in >10 % of sample size) | N | % |
| High grade fever | 1385 | 94 |
| Headache | 604 | 41 |
| Vomiting | 467 | 31.7 |
| Myalgia | 332 | 22.5 |
| Generalized body ache | 289 | 19.6 |
| Generalized weakness | 229 | 15.5 |
| Dizziness | 185 | 12.6 |
| Pain in the abdomen - Generalized | 176 | 11.9 |
| Symptoms (Seen in 1 – 10 % of sample size) | ||
| Nausea | 135 | 9.2 |
| Loose stools | 127 | 8.6 |
| Arthralgia | 103 | 7 |
| Decreased appetite | 95 | 6.4 |
| Low grade fever | 85 | 5.8 |
| Dry cough | 83 | 5.6 |
| Cough with expectoration | 80 | 5.4 |
| Backache | 78 | 5.3 |
| Melena | 45 | 3.1 |
| Retro-orbital pain | 39 | 2.6 |
| Breathlessness | 33 | 2.2 |
| Vaginal bleeding | 33 | 2.2 |
| Rash | 32 | 2.2 |
| Epigastric pain | 31 | 1.7 |
| Common cold | 25 | 1.5 |
| Gum bleeding | 22 | 1.2 |
| Decreased urination | 17 | 1.2 |
| Increased menstrual bleed | 17 | 1.2 |
| Burning micturition | 17 | 1.2 |
| Disorientation | 16 | 1.1 |
| Symptoms (<1 %) | ||
| Constipation | 15 | 1 |
| Hematuria | 14 | 1 |
| Paralysis | 9 | 0.6 |
| Loss of vision | 8 | 0.5 |
| Hiccups | 7 | 0.5 |
| Epistaxis | 7 | 0.5 |
| Throat pain | 6 | 0.4 |
| Pedal edema | 6 | 0.4 |
| Altered sensorium | 6 | 0.4 |
| Jaundice | 4 | 0.3 |
| Generalized tonic-clonic seizure | 3 | 0.2 |
| Weight loss | 3 | 0.2 |
| Abdominal distension | 3 | 0.2 |
| Hematemesis | 2 | 0.1 |
| Chest pain | 2 | 0.1 |
| Watering of eyes | 2 | 0.1 |
| Itching | 2 | 0.1 |
| Hemoptysis | 1 | 0.1 |
| Signs | N | % |
| Hypotension | 110 | 7.5 |
| Abdominal tenderness | 110 | 7.5 |
| Splenomegaly | 58 | 3.9 |
| Hepatomegaly | 56 | 3.8 |
| Petechiae | 48 | 3.3 |
| Postural drop | 41 | 2.8 |
| Lymphadenopathy | 40 | 2.7 |
| Pitting edema | 31 | 2.1 |
| Tachypnoea | 23 | 1.6 |
| Pallor | 20 | 1.4 |
| Tourniquet test | 19 | 1.3 |
| Rhonchi - Bilateral | 18 | 1.2 |
| Blanching erythematous maculopapular rash | 22 | 1.5 |
| Diffuse crepitations | 15 | 1 |
| Conjunctival congestion | 14 | 1 |
| Periorbital puffiness | 14 | 1 |
| Skin flushing | 13 | 0.9 |
| Decreased breath sounds | 11 | 0.7 |
| Deep tendon reflex decreased | 10 | 0.7 |
| Tongue coating | 9 | 0.6 |
| Signs (≤0.5%) | ||
| Dehydration | 7 | 0.5 |
| Glossitis | 7 | 0.5 |
| Disorientation | 7 | 0.5 |
| Shifting dullness | 6 | 0.4 |
| Conjunctival hemorrhage | 6 | 0.4 |
| Facial rash | 5 | 0.3 |
| Bradycardia | 5 | 0.3 |
| Macular edema | 5 | 0.3 |
| Tachycardia | 5 | 0.3 |
| Dry tongue | 4 | 0.3 |
| Icterus | 4 | 0.3 |
| Meningitis signs | 4 | 0.3 |
| Anasarca | 4 | 0.3 |
| Throat congestion | 3 | 0.3 |
| Ecchymosis | 3 | 0.3 |
| Romberg’s sign positive | 3 | 0.2 |
| Neck stiffness | 3 | 0.2 |
| Nystagmus | 2 | 0.1 |
| Sinus tenderness | 1 | 0.1 |
| Systolic murmur | 1 | 0.1 |
| Clubbing | 1 | 0.1 |
| Bell's palsy | 1 | 0.1 |
Table 4
Radiological, ECG and Endoscopic findings in Dengue Patients
| Chest X-ray | No of cases (N=324) |
| Normal | 272 |
| Pleural effusion | 30 |
| Mild pleural effusion | 7 |
| Lower respiratory tract infection / Acute respiratory distress syndrome | 7 |
| Pulmonary congestion | 5 |
| Bilateral opacity | 5 |
| Lung collapse | 3 |
| Consolidation | 2 |
| Chronic obstructive pulmonary disease | 1 |
| USG abdomen | (N=358) |
| Normal | 76 |
| Gall bladder wall edematous | 132 |
| Minimal ascites | 122 |
| Hepatomegaly | 89 |
| Fatty liver | 85 |
| Mild pleural effusion | 68 |
| Splenomegaly | 56 |
| Pleural effusion | 46 |
| Grade 1 renal parenchymal changes | 35 |
| Mild splenomegaly | 26 |
| Altered liver echotexture | 21 |
| Cholelithiasis | 19 |
| Renal calculus - urolithiasis | 16 |
| Portal hypertension | 9 |
| Prostatomegaly | 8 |
| Hydroureteronephrosis | 7 |
| Moderate ascites | 5 |
| Gall bladder sludge | 2 |
| Retroperitoneal collection | 2 |
| Pyelonephritis | 2 |
| Perinephric fluid collection | 2 |
| Urinary bladder thickening | 1 |
| Splenic cyst | 1 |
| Splenic infarct | 1 |
| Intrauterine growth restriction | 1 |
| Mesenteric lymphadenitis | 1 |
| Magnetic Resonance Imaging - Head | (N=6) |
| Normal | 2 |
| Corona radiata infarct | 1 |
| Intraventricular bleed | 1 |
| Post-viral demyelination | 1 |
| Cerebral edema | 1 |
| Computerized Tomography scan - Abdomen | (N=4) |
| Normal | 1 |
| Retroperitoneal hemorrhage | 2 |
| Inferior epigastric artery rupture | 1 |
| Computerized Tomography scan - Head | (N=13) |
| Normal | 9 |
| Cerebral edema | 2 |
| Encephalitis | 1 |
| Ischemic changes | 1 |
| Electrocardiography | (N=150) |
| Normal | 65 |
| Left ventricular hypertrophy | 22 |
| Sinus tachycardia | 21 |
| Sinus bradycardia | 16 |
| ST elevation | 13 |
| Myocardial infarction | 10 |
| Inverted T wave | 10 |
| Atrioventricular block | 6 |
| Poor R wave progression | 4 |
| QT prolongation | 3 |
| Ventricular ectopia | 2 |
| Left atrial enlargement | 2 |
| Atrial fibrillation | 1 |
| Echocardiography | (N=29) |
| Normal | 20 |
| Left ventricular hypertrophy | 5 |
| Pericardial effusion | 1 |
| Pericarditis | 1 |
| Atrial septal defect | 1 |
| Aortic regurgitation | 1 |
| Endoscopy | (N=5) |
| Normal | 4 |
| Mallory Weiss tears | 1 |
The current study, after approval from the Scientific Review and Ethical Committee, and Hospital administration, was conducted to illustrate the different clinical, epidemiological and laboratory parameters of dengue patients attending our hospitals. It aims to explore the clinical profile of dengue so to help in management of dengue patients, especially those with complications. The study was conducted on 1,474 patients who were confirmed positive for dengue fever. It was found that most cases occurred in younger adults aged between 20 and 30 years of age. The results of this study show that, in the second decade of life, dengue fever is more common in males when compared to females.
A prospective study conducted in Mangalore found that 40.6% of patients presented with hepatitis as the most common atypical manifestation [11]. A study conducted by Padhi et al. in southern Odisha, India, in 2012, found that 97.58% of patients presented with dengue fever, 2.24% with dengue hemorrhagic fever (DHF) and 0.18% presented with DSS. They also found that a majority (47.86%) of the patients presented during the month of September [12].
The present research is in accordance with other studies [13,14,15,16,17] which also show male predominance with infection. However, the exact mechanism behind this is still unknown. Most of the infections were seen in suburban areas of coastal cities. In dengue fever patients, arthralgia, myalgia, retro-orbital pain, rash, and hemorrhagic signs normally appear with a series of symptoms. In the present research, dengue fever was associated with pulmonary symptoms, gastrointestinal disorders, bradycardia, thrombocytopenia, and deranged liver function tests. Previous studies [14,16,17,18,19] (especially after outbreaks in 2010 and 2018) also document the wide range of clinical signs and symptoms associated with dengue fever, which is in accordance with our study. Most of the complications were hematological or vascular in nature, occurring in about 25% of the total number of complications, of which dengue shock syndrome (6%) and thrombophlebitis (6%) were the most frequent. The reason behind this was thrombocytopenia and perched capillary permeability. Most of our patients suffering from Acute Respiratory Distress Syndrome (ARDS) and post-dengue hematological and vascular complications required ICU admissions. It was also noted that, although dengue disease is easily prevented, prognosis worsens rapidly once complications occur (particularly if they are hemorrhagic or neurological in nature, or cavitary effusions). The total case fatality rate was 1.08% and all had succumbed following complications of dengue. It was observed that among the patients who suffered from complications, 9.4% died (Table 5). Sepsis was the major cause of mortality (56.2%) among patients suffering from complications of dengue. The neutropenia observed in our study was in agreement with the findings of Singh et al., who also noticed leucopenia [15]. This might have been a possible sequelae of myeloid progenitor cells being damaged by the virus. It was found that the risk of severity depended mostly on hematocrit, thrombocytopenia, leukopenia and LFT, similar to previous studies [13,16,17,19,20,21,22]. Serum ferritin was also found to be elevated in severe cases requiring ICU admissions in our study. This was noted to be more effective in assessing younger patients [21]. A study by Ab-Rahman et. al [23] showed that sCD163 is a good predictive marker, similar to ferritin, for assessing severity. Ascites and pleural effusion were the most frequent cavitary complications in our study. A similar incidence of ascites and pleural effusion has also been observed in dengue patients in other studies [16,17,19,20]. Myocarditis, though rare, was the most common cardiological complication and was also reported by previous studies [1]. This emphasizes the need to heed ECG changes in patients hospitalized for dengue. Our study also shows that co-infections, like leptospirosis and malaria, must be excluded before the disease progresses further. It was noted previously [1] that chikungunya often occurs concurrently with dengue. However, there is no concrete evidence to show a positive correlation between the co-existence of these infections. In recent years, the prevalence of dengue fever has increased, as an endemic infection seen increasingly in the developed parts of the world. The current study assesses the clinical features of dengue fever along with other syndromes. The study is also supported by radiological investigations. There was a statistically significant association between the use of early radiological investigation and detection and management of cavitary complications of dengue fever (χ2{1,N=1474}=8.301, p=0.0039612; p <0.05). The radiological profile showed signs such as thickened gallbladder wall, free fluid in the abdomen and hepatomegaly. This could be associated with the increased incidence (11.9%) of dengue fever patients complaining of abdominal pain. The findings of edematous and thickened gall bladder, atypical acalculous cholecystitis and hepatosplenomegaly were in concordance with the other studies [16,18]. This case series confirms the incidence of multiple central nervous system (CNS) manifestations, similar to previous reports [1,3,18,19] of dengue virus infection, and sheds light on the necessity of considering dengue as a key differential diagnosis of acute encephalitis syndrome. Furthermore, these findings imply a strong indication for radiological evaluation of patients infected with dengue as a routine clinical investigation. The strength of this study is that it has comprehensively collected the clinico-epidemiological, laboratory and radiological findings of dengue complications in an endemic area.
In conclusion, doctors in dengue-endemic areas should look for both typical (primarily thrombocytopenia) and atypical manifestations of dengue (especially neurological deficits), rule out other infections and use prompt investigations, including radiological tests and prognostic markers like serum ferritin, to manage the disease. Close monitoring of platelet count, packed cell volume (PCV) and LFT is important, in particular for patients aged below 5 years. While hyponatremia was not statistically shown to have an association with prognosis of the cases with complications, early radiological investigations (chest X-ray and USG of the abdomen) were found to have a positive association with detecting and managing complications secondary to dengue fever. Strict surveillance and assessment of atypical manifestations are needed to prevent morbidity and mortality associated with dengue.
All authors declared no conflicts of interest.
Akash Kumar- Contributed to conception, data collection, analysis, writing of original draft, editing and finalising the version to be submitted. Adarsh Sugathan- Contributed to writing the original draft, editing, and finalising the version to be submitted. Nayanatara Arun Kumar- Contributed to the conception of the study, supervised, analysed, wrote the original draft, edited, and finalised the version to be submitted. Basavaprabhu Achappa- Contributed to the supervision of the study, edited and finalised the version to be submitted. All authors have read and approved the final version of the manuscript to be submitted.
The authors thank the staff of Govt.Wenlock Hospital, Mangalore; Lady Goeschen Hospital, Mangalore and KMC Attavar Hospital, Mangalore for their support.
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request to the corresponding author.
1. Gupta N, Srivastava S, Jain A, et.al. Dengue in India. Indian J Med Res. 2012;136(3):373-390.
2. World Health Organization (WHO). Dengue hemorrhagic fever: diagnosis, treatment, prevention and control. Available at https://iris.who.int/handle/10665/41988. Accessed September 21, 2023
3. Carabali M, Hernandez LM, Arauz MJ, et al. Why are people with dengue dying? A scoping review of determinants for dengue mortality. BMC Infect Dis. 2015;15:301.
4. Adane T., Getawa S. Coagulation abnormalities in Dengue fever infection: a systematic review and meta-analysis. PLoS Neglected Tropical Diseases. 2021;15(8)
5. Rodriguez-Roche R, Gould EA. Understanding the Dengue Viruses and Progress towards Their Control. BioMed Research International. 2013; 2013:690835.
6. Gulati S, Maheshwari A. Atypical manifestations of dengue. Tropical Medicine and International Health. 2007; 12(9):1087–1095.
7. Whitehorn J, Yacoub S, Anders KL, et al. Dengue Therapeutics, Chemoprophylaxis, and Allied Tools: State of the Art and Future Directions. Rothman AL, ed. PLoS Neglected Tropical Diseases. 2014; 8:e3025.
8. Yacoub S, Mongkolsapaya J, Screaton G. Recent advances in understanding dengue. F1000Research. 2016; 5: F1000 Faculty Rev-78.
9. Gordon A, Kuan G, Mercado JC, et al. The Nicaraguan Pediatric Dengue Cohort Study: Incidence of Inapparent and Symptomatic Dengue Virus Infections, 2004–2010. Pettigrew MM, ed. PLoS Neglected Tropical Diseases. 2013; 7:e2462.
10. Pal S, Dauner AL, Valks A, et al. Multicountry Prospective Clinical Evaluation of Two Enzyme-Linked Immunosorbent Assays and Two Rapid Diagnostic Tests for Diagnosing Dengue Fever. Munson E, ed. Journal of Clinical Microbiology. 2015; 53(4):1092-1102.
11. Nimmagadda SS, Mahabala C, Boloor A, et al. Atypical Manifestations of Dengue Fever (DF) – Where Do We Stand Today? Journal of Clinical and Diagnostic Research: JCDR. 2014; 8:71-73.
12. Padhi S, Dash M, Panda P, et al. A three year retrospective study on the increasing trend in seroprevalence of dengue infection from southern Odisha, India. The Indian Journal of Medical Research. 2014; 140:660-664.
13. Moraes GH, de Fátima Duarte E, Duarte EC. Determinants of mortality from severe dengue in Brazil: a population-based case-control study. The American journal of tropical medicine and hygiene. 2013;88:670
14. Vijay J, Anuradha N, Anbalagan VP, et al. Clinical presentation and platelet profile of dengue fever: a retrospective study. Cureus. 2022 ;14. e28626
15. Singh J, Dinkar A, Atam V, et al. Awareness and outcome of changing trends in clinical profile of dengue fever: a retrospective analysis of dengue epidemic from January to December 2014 at a tertiary care hospital. J Assoc Physicians India. 2017;65:42-46.
16. Deshwal R, Qureshi MI, Singh R. Clinical and laboratory profile of dengue fever. J Assoc Physicians India. 2015;63:30-32.
17. Khurshid A, Syed A, Shafie AA, et al. Clinical manifestations and laboratory profile of dengue fever among the patient's general hospital, Penang. Arch Pharm Pract. 2010; 1:25-29.
18. Laul A, Laul P, Merugumala V, et al. Clinical profiles of dengue infection during an outbreak in Northern India. Journal of tropical medicine. 2016; doi: https://doi.org/10.1155/2016/5917934
19. Khan MY, Venkateshwarlu C, Sandeep N, et al. A study of clinical and laboratory profile of dengue fever in a tertiary care hospital, Nizamabad, Telangana State, India. Headache. 2016;115:76-7.
20. Mia MW, Nurullah AM, Hossain A, et al. Clinical and sonographic evaluation of dengue fever in Bangladesh: a study of 100 cases. Dinajpur Med Col J. 2010;3:29-34.
21. van de Weg CA, Huits RM, Pannuti CS, et al. Hyperferritinaemia in dengue virus infected patients is associated with immune activation and coagulation disturbances. PLoS Negl Trop Dis. 2014;8:e3214
22. Priya SP, Sakinah S, Sharmilah K, et al. Leptospirosis: molecular trial path and immunopathogenesis correlated with dengue, malaria and mimetic hemorrhagic infections. Acta tropica. 2017;176:206-23.
23. Ab-Rahman HA, Rahim H, AbuBakar S, Wong PF. Macrophage activation syndrome-associated markers in severe dengue. International Journal of Medical Sciences. 2016;13:179