Watching Brief
| Date of first report of the outbreak | The outbreak was first reported in the EPIWATCH system on September 19th, 2022 (1) |
||||||||||||||||||||||||||||||||||||||||||||||||
| Disease or outbreak | Ebola Virus Disease (EVD), Sudan Virus Disease (SUVD) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Origin (country, city, region) | Madudu sub-country, Mubende district, Uganda |
||||||||||||||||||||||||||||||||||||||||||||||||
| Suspected Source (specify food source, zoonotic or human origin or other) | As of September 20th, the World Health Organization (WHO) were working closely with the Ministry of Health, Uganda, to identify a source of the outbreak (2). There have been no additional details provided by the WHO or the Ministry of Uganda identifying a source of the outbreak. The virus was likely circulating at least three weeks prior to the identification of the first case (3), suggesting that finding a source for the outbreak may prove to be difficult and not a current priority given the ongoing rapid transmission and high level of risk to the community. However, the source is likely an infected animal (4). |
||||||||||||||||||||||||||||||||||||||||||||||||
| Date of outbreak beginning | This outbreak was declared on September 20th, 2022 (4). However, given there are suspected cases who were infected at the start of September, this more so represents when the Ministry of Health in Uganda identified the outbreak as opposed to representing the date the outbreak began according to epidemiological terms. The first confirmed case was a 24 year old male who presented to a private clinic on September 13th and then a regional hospital on September 15th (4). |
||||||||||||||||||||||||||||||||||||||||||||||||
| Date outbreak declared over | As of October 11th, the outbreak is still ongoing and likely to continue given there has been prior unidentified transmission throughout various districts (2, 5, 6). |
||||||||||||||||||||||||||||||||||||||||||||||||
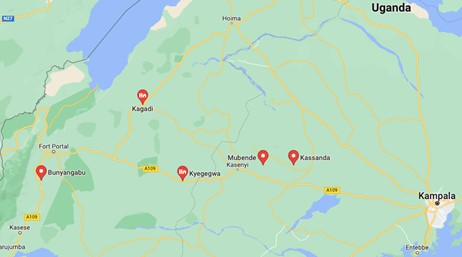
| Affected countries & regions | As of October 11th, the cases were dispersed across five towns in Uganda; Mubende, Kyegegwa, Kassanda, Kagadi and Bunyangab (6). The now suspected cases, some of whom died of the disease, from the weeks prior to the outbreak starting, were from villages in the Madudu (where the first confirmed case was from) and Kiruma sub-counties of Mubende district (4). As you can see in Figure 1 below, the disease has spread mainly to Western Uganda, with Kampala in the East.
Figure 1
Uganda SUVD outbreak (7) |
||||||||||||||||||||||||||||||||||||||||||||||||
| Number of cases (specify at what date if ongoing) | As of October 11th, there are a total of 43 confirmed cases and 9 total deaths (5, 6). Of all the cases, (confirmed, suspected and probable) there are a total of 63 with 29 deaths (6, 8). |
||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical features | The incubation period for EVD ranges from 2-42 days after contact with the virus, with symptoms usually exhibiting between 8 to 21 days, with 21 days frequently reported as the average (9, 10). The virus is not transmissible until symptoms appear; however, patients remain infectious whilst the virus is detectable via polymerase chain reaction (PCR) (4). Disease progression typically presents with ‘dry’ non-specific symptoms including fever, aches and pains, sore throat, and fatigue then progressing to ‘wet’ symptoms, including diarrhea and vomiting (9). Such non-specific clinical manifestations of the disease may mimic other infectious diseases including typhoid, malaria, and meningitis. Clinical progression from dry to wet symptoms leads to severe lethargy, loss of appetite and unexplained haemorrhaging, internal and external bleeding, and bruising (9). End-stage disease cases exhibit red eyes, skin rash and often hiccups (9). Infected individuals can develop multi-organ failure with impaired kidneys and decreased liver function whilst laboratory findings include low white blood cells, decreased platelet counts and elevated liver enzymes (11). Although this course of illness is rare, it can be fatal if not treated promptly. Survival is dependent on expedited clinical care, including hydration and electrolyte replacement, and the patient’s immune response. Survivors develop neutralizing antibodies that have shown to be detectable for up to 10 years post recovery, with these survivors having some protective immunity from the strain of EVD that sickened them (12). The clinical features in the current outbreak have been described in the first report from Mubende district, Uganda, where the index case was identified in a 24-year-old male on September 11th who exhibited wide-ranging symptoms including high-grade fever, tonic convulsions, blood-stained vomit and diarrhoea, loss of appetite, pain while swallowing, chest pain, dry cough and bleeding in the eyes. He later succumbed to the illness on September 19th (4). All additional confirmed, suspected, and probable cases have been reported to exhibit EVD symptomology (2, 3, 5, 6, 8, 12). |
||||||||||||||||||||||||||||||||||||||||||||||||
| Mode of transmission (dominant mode and other documented modes) | Transmission to humans occurs when people come into close contact with bodily fluids, including blood or secretions, and body parts or the carcass of an infected animal (4). The animal is often a fruit bat, chimpanzee, gorilla, monkey, forest antelope or porcupine (4). Following this, the principal mode of transmission in human outbreaks is from person-to-person involving direct or close contact with bodily fluids including blood, semen, saliva, tears, stool, breast milk or other secretions and through skin contact (13, 14). Droplet and bodily fluid transmission are the most common routes, however EVD may also be transmitted through fomites (15), with research finding that live virus can be found in dried blood (14) or on glass after numerous days (16). However, further research is required to understand if the virus can be acquired off contaminated surfaces. The virus can transmit via soiled materials such as gloves, bed linen or clothing (15). Community deaths and unsafe burials, including the handling of sick patients and dead bodies, have been documented as driving rapid increases in infection rates during past Ebola outbreaks (17). Such practices have already been reported as occurring relative to the first few deaths in this current outbreak, given the causes of death were initially a mysterious illness (3, 17). Transmission in the absence of direct contact, presumably through aerosols, as well as through needlestick injury has also been described (18). |
||||||||||||||||||||||||||||||||||||||||||||||||
| Demographics of cases | As of September 27th, of the total confirmed and suspected cases, 62% were female and 38% were male (4). The median age of cases were reported to be 26 years ranging from 1 year to 60 years (4). However, as case numbers continued to rise, the reporting of suspected and probable cases and demographics ceased (5). The affected districts are home to over 1.3 million people, with the least dense area being the towns of Madudu and Kagadi with a population of approximately 41, 000 (19, 20). There is roughly an even distribution between male and women in all areas and a young population with a mean age of 15.9 years (19-23). |
||||||||||||||||||||||||||||||||||||||||||||||||
| Case fatality rate | The case fatality rates (CFR) of past outbreaks of EVD led by SUVD have been between 41% and 100% (4). The current CFR is 25.6% based off the confirmed cases. However, it is higher at 46% counting suspected and probable cases as well (8). |
||||||||||||||||||||||||||||||||||||||||||||||||
| Complications | Although a complication rate for EVD specific strains is not available post EVD syndrome, survivors complain of fatigue, vision loss, hearing loss, headaches, memory loss, sleep disorders, mood disturbances, abdominal pain, amenorrhea, miscarriages, enthesitis and arthralgias (24, 25). Ongoing clinical sequelae have been reported in cases for up to 2 years after acute disease (26). Of note, a Scottish nurse who contracted EVD and recovered, suffered relapsing illness with meningitis, though later recovered after many months of illness (27). However, the clinical symptoms aren’t well characterised in African countries and are often self-reported by survivors with further research required on populations who have experienced EVD illness (28). As of October 11th, in the current outbreak, 27 deaths have been reported, 9 confirmed with SUVD (5, 8). |
||||||||||||||||||||||||||||||||||||||||||||||||
| Available prevention | There is no approved vaccine for SUVD (2). There is one approved EVD vaccine for the Zaire strain and although this vaccine has proven to be very effective in managing Zaire outbreaks using ring vaccination, it is likely not effective for the Sudan strain (4). However, there are numerous vaccine candidates for SUVD, of note vaccines being developed by Johnson and Johnson, at Oxford University and at the Sabin Vaccine Institute (4). The preventative strategies used for SUVD are consistently recommended for EVD (29). The following recommendations should be followed when any individual is in an area where EVD may be present:
Healthcare workers and other patients in healthcare facilities are at an increased risk of acquiring the infection when there is a patient with EVD present (4). To prevent spread of the disease to other patients and staff, patients with confirmed or suspected EVD should be managed in a separate isolation tent with their own care delivery kits. Healthcare staff attending to the patients need to be trained in infection control practices and the proper use of personal protective equipment (PPE), and there should be separate screening and triage areas at each facility (4). |
||||||||||||||||||||||||||||||||||||||||||||||||
| Available treatment | Treatment for acute EVD is resource dependent with fundamental principles of isolation, monitoring and supportive care the recommended approaches (30). In resource limited settings patients are assessed and triaged based on volume status, evidence of organ failure and ability for self-care (31). If hypovolemic and not in organ failure, self-management is the focus of treatment in settings where intravenous fluid management options are limited (30). Pharmacological support with antiemetics, antidiarrheals and oral rehydration solutions are offered to counter gastrointestinal loses with acute EVD (31, 32). Post-exposure treatment with antiviral therapeutics, plasma or whole blood transfusions and triple monoclonal antibody cocktails have been trialled across past outbreaks of EVD within African countries, however, results from these studies varied and were not statistically significant (30). Newer combination therapy with remdesivir and monoclonal antibodies showed promising results as a viable treatment against SUVD, when six days after therapy commencement an 80% protective level was achieved in rhesus macaques (33). |
||||||||||||||||||||||||||||||||||||||||||||||||
| Comparison with past outbreaks | There have been 7 previous recorded outbreaks of SUVD, four in Uganda (years 2000, 2011, 2012 and 2012-2013), and three in Sudan with the first outbreak occurring in Sudan in 1976 (years 1979 and 2004) as represented in Table 1 (2). As represented below, the current outbreak has 43 confirmed cases and is the second largest outbreak of SUVD in Uganda, and the third largest recorded outbreak across both countries (34). This outbreak also has the lowest official CFR of SUVD; however, this is likely inaccurate given the number of suspected and probable cases. It is important to note the recent 2011 outbreak in Uganda had only one reported case who died from the disease (100% CFR). Table 1 Recorded SUVD outbreaks (6, 8, 34)
*Outbreak is ongoing, and these figures are subject to change The stark difference between the management of the first case in the 2011 outbreak should be noted; in 2011, healthcare workers were immediately suspicious that Ebola could be the cause of infection when the first case presented to hospital (35). The case was immediately isolated and managed with infection control precautions and underwent rapid testing (35). In this current outbreak, there is no mention among available sources that the prior cases who are now suspected cases were treated as if they may have had a viral haemorrhagic fever (4). Further, these initial suspected cases visited numerous health facilities whilst infectious, including the first confirmed case who visited 2 private clinics across 2 days and then was referred to the hospital with a suspected viral haemorrhagic fever where he underwent testing (4). The delayed response in suspecting EVD as a possible cause of illness likely has aided in ongoing transmission across Uganda and into five districts. |
||||||||||||||||||||||||||||||||||||||||||||||||
| Unusual features | Although Uganda has had experience with managing past EVD outbreaks, this outbreak presents specific challenges due to the rarity of this strain, one of the reasons as to why the WHO have classified the risk of serious public health impact as high (36). Adding to this high-risk classification is the unidentified transmission in prior weeks, the delayed infection control response of clinicians and health authorities and the lack of available vaccines for prevention of disease (4). Thus far, ten healthcare workers have been infected and sadly four deaths recorded where transmission occurred in the workplace (6). The CFR for this strain is already high, however in this landscape of potentially ongoing community transmission, the CFR and the burden on the health care system may well be magnified. Uganda’s last outbreak of this disease was ten years ago, and the driving causes behind its current re-emergence are unknown. The cause is hard to determine given the source of the outbreak has not been identified and the primary focus is now on emergency response, active surveillance, and health system support (4). The geographical location of the affected districts presents concerns for the Government and health authorities (12). The affected districts are dispersed across 75 miles of a highway which acts as a main route between the Ugandan capital Kampala and neighbouring country, the Democratic Republic of the Congo, which has just declared its most recent EVD outbreak over (37). The outbreak is also near a popular goldmine that has a very mobile workforce (4). The risk of spread to other areas of Uganda and other countries is high (4). Simultaneously, the Ugandan health system is co-managing outbreak response of anthrax, COVID-19, Rift Valley fever and Yellow fever, along with other emergencies such as flooding and food insecurity (4). Another large outbreak of Ebola could have extensive negative effects across numerous industries and influence income. |
||||||||||||||||||||||||||||||||||||||||||||||||
| Critical analysis | On the back of previous devastating outbreaks of Zaire and SUVD, Uganda's health infrastructure was left underdeveloped with limited financial resources in 2010 (38, 39). A viral haemorrhagic fever surveillance programme was then established, which may have contributed to the successful control of the 2011 SUVD outbreak which reported only one confirmed case with no onward transmission (35, 38). Uganda has developed and implemented epidemic preparedness activities whilst learning from previous mistakes to prevent EVD cross-border transmission by enabling timely detection, investigation, and response to confirmed EVD outbreaks in the country (40). However, it is possible infections of SUVD have gone unreported in the current outbreak due to barriers affecting access to healthcare in rural communities owing to urban bias, social determinants of health, and poor transportation (41). Of note, two probable deaths from the first two weeks of September in Mubende were reported as likely be attributed to SUVD (4), highlighting concerns that the current outbreak may be more widespread with no identified index case. Whilst preparedness programs are in place, key barriers remain including the political instability, rebel activities and community mistrust in neighbouring Democratic Republic of the Congo leading to a large influx of people crossing the border into the country putting a strain on border screening (40). Furthermore, fatigue in preparedness efforts may have set in whilst a combination of inadequate funding and resource allocation to cover high-risk districts coupled with poor public messaging could be contributing to the current outbreak. Adding to Uganda’s worries is the fractured healthcare system. Whilst healthcare workers are expected to care of the sick, ongoing disputes between the Uganda Medical Association and Health Ministry over risk allowance and compensation for caring for SUVD patients is continuing to hamper control efforts (42). Whilst simultaneously battling COVID-19 and ongoing natural and manmade disasters the healthcare system will require additional support and resources from the global community. Another challenging aspect to this current outbreak is population mobility, where individuals incubating the virus who travel frequently unknowingly spread SUVD (43). The current outbreak was reportedly detected within a community with an active gold mine (3). Mobility among these miners and traders will likely be high. With the declaration of an outbreak in an area of high occupancy, miners may flee the area whilst already incubating the disease and seed the virus into surrounding districts and across borders into neighbouring countries. As such four additional districts across 240 kilometres in Uganda have reported cases of SUVD (3). Epidemiological linkage between these cases and deaths to confirmed cases needs to be rapidly established to track the evolution of the current outbreaks spread. Concerningly, an international airport located in Entebbe is within a short distance to Kampala, where although no cases have been reported if cases continue to spread, there is risk of international transmission beyond Uganda. To control ongoing transmission, confirmed cases require isolation in specialist isolation facilities with sufficient PPE used by trained healthcare workers experienced in airborne precautions. Furthermore, clinicians require ongoing refresher training on surveillance, detection, and outbreak management. It has been reported that a treatment facility near Kampala closed due to six medical staff becoming infected with the virus and subsequently 34 employees went on strike (44). This has been reported in previous EVD outbreaks across the African continent with the closure of facilities leading to community transmission and increased deaths (45, 46). Furthermore, most recent reporting suggests ten healthcare workers are known to be infected and four have sadly died (6). These healthcare workers may not have had adequate infection, prevention and control training coupled with adequate PPE to protect themselves before succumbing to the disease. As community transmission continues to rise, so to do the numbers of close contacts that have been identified for follow-up, with the most recent totalling 882 (8). It remains unknown if the Ugandan Government can control community spread by enacting public health measures to follow-up close contacts and enforce strict isolation. A recent report indicated resources were already stretched with no capacity to accommodate those needing isolation (47). Whilst it is believed the natural reservoirs of EVD are thought to be various African fruit bats (48), the natural reservoirs for SUVD remain unknown. Therefore, community engagement and education campaigns are critical to inform people of the risks of contact with animals who may be infected with SUVD. Furthermore, whilst no effective vaccination is available to treat SUVD there is an urgent requirement to develop treatments and begin trialling of current vaccine candidates. In short, the current outbreak appears to have spread quickly across five districts due to highly mobile members of communities travelling for employment, to source food and see their families. The increased number of confirmed and probable cases over a relatively short period of time and growing close contacts is concerning for outbreak control. A lack of viable vaccines and poor health infrastructure with limited finances seem to be contributing factors to the current outbreak. Whilst the true natural reservoir and how spill over to human hosts occurs remains unknown (49) these outbreaks of EVD will continue to occur in African countries where the virus remains endemic. |
||||||||||||||||||||||||||||||||||||||||||||||||
| Key questions | 1. In the absence of a licenced and effective vaccine with efficacy data or therapeutics for prevention and treatment for SUVD, how can the current outbreak be contained in Uganda? 2. How quickly will the vaccine trials be prioritized as a key component of managing and controlling the outbreak, amid the ongoing COVID-19 pandemic and with recent rapid advancements in vaccine development? 3. Would ring vaccination with the “Johnson and Johnson” adenovirus vector-based two dose schedule vaccines provide any systemic protection during the current outbreak? 4. Was the index case the true index case? As all chains of transmission can’t be tracked within surrounding districts and the true effect of virus seeding into surrounding districts remains unknown. 5. Considering the Democratic Republic of Congo declared their EVD outbreak over on September 27th (37), how will surrounding countries at risk of SUVD transmission prepare for another outbreak of a rarer strain? Can preventative measures be enforced to stop spread of the current outbreak seeding into neighbouring countries or across international borders? 6. Whilst the DRC shares a border with Uganda and high mobility between nations occurs, could the recent outbreak in the DRC be genetically linked to the current outbreak in Uganda? |