Watching Brief
| Date of first report of the outbreak | A study published on 4 August 2022 was the first to report on human cases and disease emergence [1]. |
| Disease or outbreak | Langya henipavirus (LayV) |
| Origin (country, city, region) | LayV was first detected as a phylogenetically distinct henipavirus in Qingdao, Shandong province, during an active surveillance study conducted between April 2018 – August 2021 in Shandong and Henan provinces, which recruited and monitored patients with acute fever and history of animal exposure [1]. |
| Suspected Source (specify food source, zoonotic or human origin or other) | LayV is reportedly zoonotic, with shrews suspected to be the natural reservoir host [1]. Specifically, LayV was detected in Ussuri (Crocidura lasiura) and Asian lesser (Crocidura shantungensis) white-toothed shrews (LayV RNA positivity rates of 52.1% and 20.0% respectively) [1]. LayV was also detected in a northern red-backed vole (Myodes rutilus), striped field mice (Apodemus agrarius) and house mice (Mus musculus), though positivity rates were low (0.6-1.3%) [1]. Seropositivity rates in domestic goats and dogs were 2.0% and 5.0%, respectively [1]. Patients were provided standard questionnaires as part of epidemiological investigations, which included exposure history prior to illness onset. However, the study did not specify the types of animals with which patients had contact, nor whether this question was asked of patients [1]. |
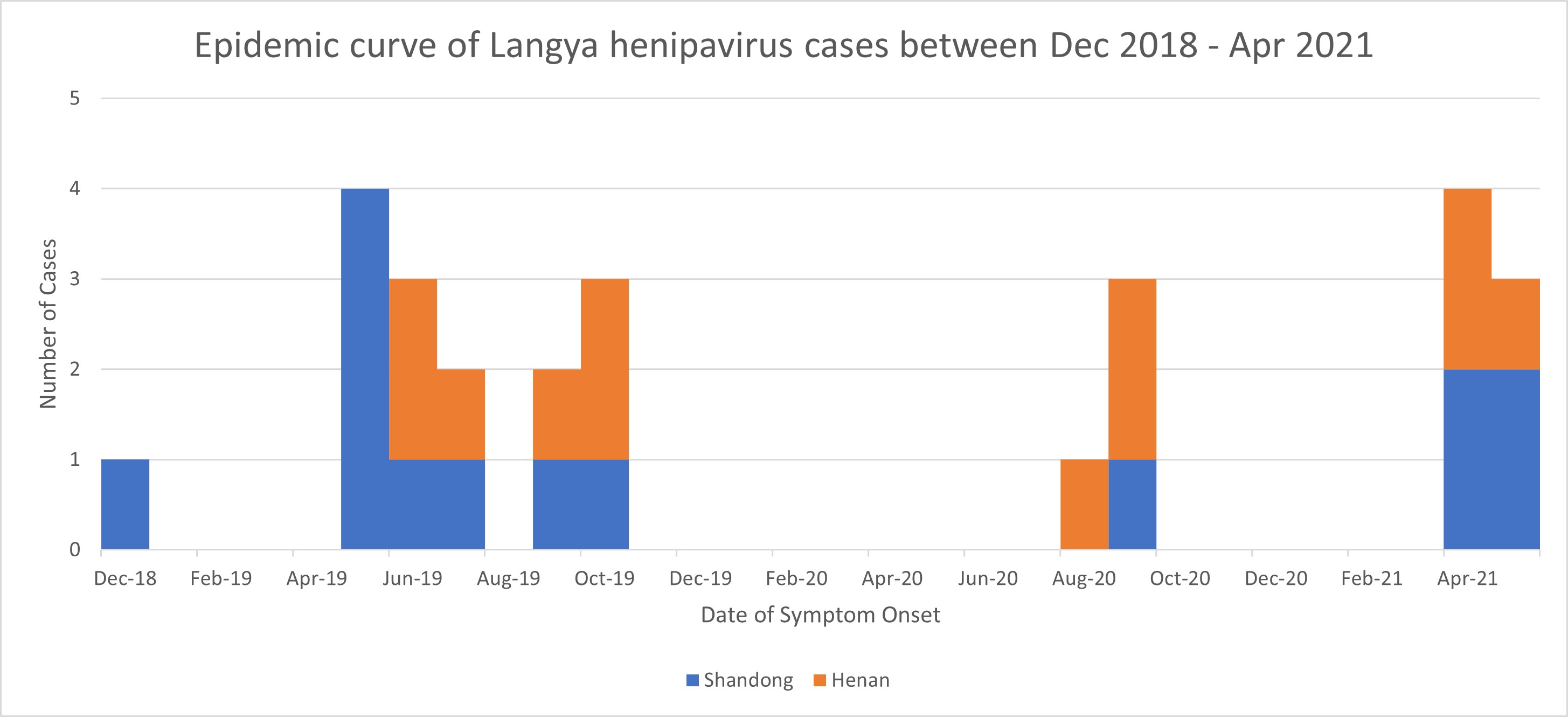
| Date of outbreak beginning | The first patient was reported in December 2018, after presenting to a sentinel hospital for treatment. |
| Date outbreak declared over | Although the study by Zhang et al. (2022) concluded in August 2021 [1], disease surveillance is now ongoing [2, 3]. |
| Affected countries & regions | China (Shandong and Henan provinces) |
| Number of cases (specify at what date if ongoing) | 35 cases (last patient identified in May 2021) |
| Clinical features | Clinical features were reported for 26 patients in which LayV was the only potential pathogen detected. The other patients had co-infection with: severe fever with thrombocytopenia syndrome virus (n=6), haemorrhagic fever with renal syndrome virus (n=2), and influenza (n=1). Reported clinical features (and case percentages) were:
|
| Mode of transmission (dominant mode and other documented modes) | Sporadic zoonotic transmission is suspected, given the history of animal exposure in LayV cases and lack of temporal/spatial clustering or epidemiological linkage [1]. It is currently unknown whether patients were infected directly or indirectly from shrews or via an intermediate host [4]. No infection occurred among 15 close contacts of nine patients, though sample size was insufficient to confirm or deny potential human-to-human transmission [1]. Further research is required to understand LayV transmission. |
| Demographics of cases | Of 26 patients with only LayV infection:
|
| Case fatality rate | No deaths (CFR = 0%) have been reported to date. |
| Complications | Reported complications were:
|
| Available prevention | No information is currently available regarding LayV prevention. However, LayV is closely related to other henipavirus species, Mojiang virus (MojV) [1], Hendra virus (HeV) and Nipah virus (NiV) [5]. Little is known regarding MojV and whether it infects humans, thus there is no information available regarding MojV prevention or treatment. However, prevention measures for HeV/NiV, which are zoonotic, include avoiding direct contact with infected animals and their secretions/excretions, and using appropriate personal protective equipment during close contact. It would likely be reasonable to use similar precautions for LayV. |
| Available treatment | Little is currently known regarding LayV treatment. Although Zhang et al., (2022) collected information regarding treatment regimens for the affected patients, these details were not provided [1]. However, treatment for similar infections with HeV/NiV is primarily supportive [6,7]. Some antivirals are under investigation: ribavirin has been used as a compassionate therapy, though results regarding efficacy are conflicting [8,9]; favipiravir provided protection for hamsters challenged with a lethal NiV dose [10]; and a recent phase I clinical trial found monoclonal antibody m102.4 intravenous infusions well-tolerated and safe [6,11]. |
| Comparison with past outbreaks | LayV is phylogenetically most closely related to MojV, another henipavirus species first identified in Yunnan Province, China [12]. In 2012, six labourers working in an abandoned mine fell ill with severe pneumonia of unknown cause, with three deaths [12,13]. Later, researchers found three rats (Rattus flavipectus) in the same cave with positive MojV samples, though the virus could not be isolated [12]. Furthermore, the cause of illness in the labourers has not been officially identified [12-14]. Thus, it is currently unknown whether MojV can infect humans [15]. However, genomic sequencing indicates MojV has different cell-entry mechanisms and lack of cross-reactivity with HeV, NiV and Ghanaian bat henipavirus [15,16]. Fruit bats (particularly Pteropus) are the natural reservoir host for NiV and HeV and may infect humans or other mammals [5,17-19]. Humans typically become infected via close contact with an intermediary or amplifier host (eg. NiV from pigs, HeV from horses) [17]. NiV transmission may also occur via close contact between humans, contaminated food, and possibly fomites; airborne transmission has not been reported [7,17,20-24]. NiV was first identified during an outbreak across Malaysian pig-farms in 1998-1999 [8]. Infection typically involves fever with encephalitis and/or respiratory involvement, with CFR estimated between 40-75% [7,9]. HeV was first identified in Queensland, Australia in 1994 [17]. To date, seven people in Queensland have been infected, all following direct contact with infected horses and their respiratory secretions/bodily fluids, with four deaths [17,25]. Infection may cause mild influenza-like illness to fatal respiratory or neurological disease [6]. A commercially available HeV animal vaccine provides immunity in horses for up to six months [26]. |
| Unusual features | The mostly mild symptoms, lack of evident connection, and sporadicity of LayV cases appear reassuring. However, cases without recruitment criteria may have been missed. Furthermore, gingival bleeding, due to thrombocytopaenia, has been suggested to signal serious illness and present early indication of infection [27]. Since thrombocytopaenia is associated with increased mortality risk in ICU settings [28,29], LayV infection could have significant implications for already critically ill patients. It is currently unknown how the LayV patients were infected, however, cases predominantly occurred between May-October (Figure 1), coinciding with breeding season for most shrew species [30-33], potentially increasing exposure. Though no cases were detected between Oct 2019 to Aug 2020, this could have been impacted by COVID-19 restrictions in early 2020 [34]. Additionally, seropositivity in domestic animals, though low [1], may warrant further investigation given their proximity to humans.
Figure 1
Figure 1. Epidemic curve of Langya henipavirus cases in Shandong and Henan provinces between Dec 2018 – Apr 2021. |
| Critical analysis | LayV has drawn further attention to the impact of emerging zoonoses. Though 75% of emerging infectious diseases (EIDs) are zoonotic [35], LayV is of great interest as it is a zoonotic henipavirus, like HeV and NiV. Both HeV and NiV are on the US “Select Agents and Toxins List” [36], while NiV is considered to have pandemic potential as it exhibits both zoonotic and human-to-human transmission [37]. Although most LayV cases were mild, ongoing surveillance is crucial for gathering further information regarding transmission and pathogenesis [2,3]. Similarly, continued surveillance and analysis of MojV may be useful given their phylogenetic similarity [1]. EID identification may be challenging as cases may be sporadic without obvious links. Though the case definition used in the LayV study appears broad (acute fever and recent history of animal exposure) [1], fever may not be the main symptom of LayV, nor occur in all cases, thus additional cases could have been missed. Concurrently, it is also important to ensure that infected cases are identified efficiently without excessive false positives. Ongoing surveillance efforts should therefore carefully consider case definitions and testing criteria to achieve appropriate sensitivity and specificity. If LayV is zoonotic, environmental factors could have influenced its emergence. For example, shrews have diverse habitats, are active day and night, and exhibit more ground-surface activity in summer than winter [38-41], which may increase potential human contact with shrews when outdoors. Anthropogenic activities may influence human-wildlife interactions and increase the potential for zoonotic spillover [42-46]. Since most LayV cases were in farmers, it is worthwhile considering whether seasonal or geographical factors or anthropogenic activities (e.g. relating to farm settings and/or activities, amount and/or timing of outdoor exposure, etc.) might have contributed to transmission. Though LayV cases were detected across two eastern provinces, cases could have occurred elsewhere, as shrews exhibit wide geographical distribution, including north-eastern and southern China [47,48], thus warranting expansion of surveillance within China and possibly beyond [49]. EID detections have also largely occurred post-emergence during case-management of human infections [50]. However, by this time, significant morbidity or mortality may have already occurred [50]. Therefore, systems for estimating risks of infectious disease emergence, based on context-specific risk factors, may be useful in providing early warnings of zoonotic spillover [50,51]. Greater understanding of LayV transmission and pathogenesis will require vigilant surveillance of animal and human health, and knowledge of complex human and animal ecological relationships, based on effective communication between provinces in China and neighbouring countries. EIDs can pose significant risks to public health, with unique challenges for health systems worldwide relating to their detection, identification, and management. LayV could present a substantial threat to human health, given its potential for zoonotic transmission, its possible widespread geographic host distribution, and relatedness to other more severe henipavirus species. Thus, further investigation into LayV is crucial to better prepare public health systems responses to the potential health impacts of this emerging disease. |
| Key questions |
|