Watching Brief
| Date of first report of the outbreak | 09 May 2022 [1] |
| Disease or outbreak | Indonesia is currently experiencing a foot-and-mouth disease (FMD) outbreak in livestock, caused by the foot-and-mouth disease virus (FMDV), an aphthovirus species (family Picornaviridae) [2]. |
| Origin (country, city, region) | FMD is believed to have first been described in the 16th century in cattle in Italy [3]. FMD is enzootic in Asia, South America, Africa and the Middle East, with sixty-nine countries recognised as FMD-free by the World Organisation of Animal Health (WOAH) [4]. FMDV has seven serotypes: O, A, C, SAT 1, SAT 2, SAT 3, Asia 1 [5]. Serotypes O and A are most commonly identified globally, occurring in Africa, Asia and South America [6-8]. The SAT and Asia 1 serotypes are generally restricted to Africa and Asia, respectively, though incursions into other regions have occurred [9]. Serotype C, last documented during outbreaks in Brazil and Kenya in 2004, appears to be no longer circulating in livestock and is possibly extinct, though there are concerns of iatrogenic re-introduction with vaccines through laboratory escapes or improper inactivation [10,11]. The current Indonesian outbreak is due to FMDV serotype O (O/ME-SA/Ind-2001e sublineage) [7]. |
| Suspected Source (specify food source, zoonotic or human origin or other) | Though inconclusive, the source is suspected to be illegal importation of animals [1,12], perhaps from nearby countries where Serotype O is endemic [7]. |
| Date of outbreak beginning | The first case is suspected to have occurred in cattle on 12 April 2022 in Jawa Timur province [1]. WOAH confirmed 3,496 cases in Jawa Timur and Aceh provinces on 6 May 2022, and suspended Indonesia’s FMD-free status with effect from 12 April 2022 [4,13,14]. |
| Date outbreak declared over | Ongoing as of 05 September 2022. |
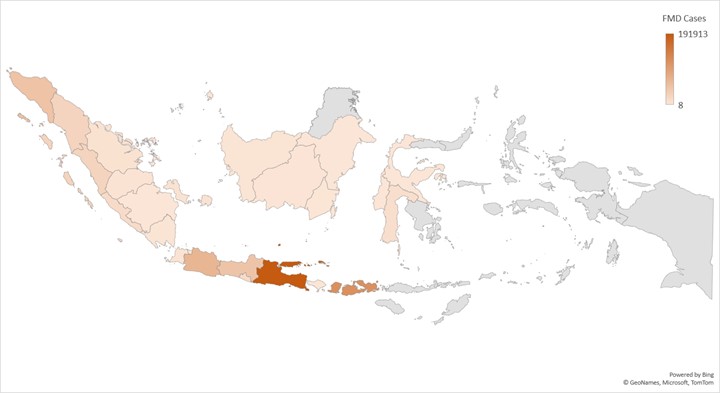
| Affected countries & regions | Indonesia (26 of 37 provinces as of 3 Nov 2022) (Figure 1)
Figure 1
Heatmap of FMD livestock cases by province in Indonesia Data sourced from the Ministry of Agriculture of the Republic of Indonesia, 2022, FMD Outbreak Management and Prevention Information. https://crisiscenterpmk.ditjenpkh.pertanian.go.id/ [15] |
| Number of cases (specify at what date if ongoing) | As of 3 November 2022, out of 54,767,135 livestock, there have been 570,137 cases (morbidity rate = 1.04%), 9,785 deaths (mortality rate = 0.02%), 12,650 culled, and 5,199,595 vaccinated. Most cases have occurred in cattle (94.27%), followed by buffalo (4.56%), goats (0.80%), sheep (0.36%), and pigs (0.02%) [15]. |
| Clinical features | Clinical features may appear within 2-14 days after infection, with severity and prevalence varying across host species. A study on the 2010-2011 FMD epidemic in South Korea reported the following clinical manifestations [16-20]:
|
| Mode of transmission (dominant mode and other documented modes) | Transmission occurs typically via direct contact with infected animals, or indirect contact with their excretions/secretions, raw meat products, or fomites (e.g. contaminated animal feed and water) [16,17]. Infected animals may shed virus during the incubation period, which is typically 2-14 days [17,21]. FMDV may survive for months under favourable environmental conditions [17]. Less frequently, aerosol transmission can occur over short and long distances, influenced by host species (e.g. aerosol emissions are much higher in swine compared to sheep and cattle [22-24]), number/location of transmitting animals, topographical and meteorological conditions, and FMDV serotype [17,23,25]. Live FMDV may persist in the oropharynx of some ruminants, known as “carriers” [26], presenting a low but possible contagion risk [27]. The presence of domestic carrier animals has important implications for FMD-free countries in terms of their ability to maintain FMD-free status and engage in international trade. However, carrier persistence and transmission mechanisms are unclear, and evidence for transmission under natural conditions is inconsistent [26,28-30]. Since carriers and non-carriers appear to exhibit similar viral shedding in saliva and nasal fluids, it is suggested that ongoing transmission from carriers may occur when oropharyngeal cells are damaged [26,31]. Highly potent vaccines may reduce carriage prevalence, unless exposure occurs soon after vaccination [5]. FMD is not considered a risk to human health. FMDV primarily infects animals and is not readily transmissible to humans as it crosses the species barrier with difficulty [4,16,32]. Though human FMD cases have been reported, they have been mild, extremely rare, and linked to close contact with infected animals or consumption of their unprocessed products [6,32-34]. |
| Host species | FMD affects cloven-hoofed mammals (artiodactyla), including domesticated ruminants, pigs and about 70 wildlife species [5,6,35]. The global FMD prevalence in livestock is estimated to be 77% [4]. Cattle are considered to be most susceptible to infection, particularly via airborne routes as they inhale larger volumes of air [5,17,33]. FMD is generally more severe in cattle and pigs, and milder in Asian water buffalo, sheep and goats [6,17]. Wild African buffalo, with subclinical infections and possible persistence of more than five years, may present a potential reservoir for transmission to domestic cattle [6,16,17]. Cattle usually remain carriers for up to six months, whereas pigs are unlikely to become carriers [17,36]. |
| Case fatality rate | CFR is low in adults (1-5%), however may be high (≥20%) in young animals [3,17-19,37]. |
| Complications | The following complications may occur, with potentially significant economic implications [19,38-44]:
|
| Available prevention | Inactivated virus vaccines are available. Routine vaccination is used in countries to maintain “FMD-free with vaccination” status and emergency, high potency vaccines are used during outbreaks where FMD is endemic [16]. However, neither natural nor vaccine-induced immunity against one FMDV serotype provides cross-protection against other serotypes [17]. Thus, vaccines are commonly prepared from at least two different serotypes to provide broad antigenic coverage. However, immunity only lasts approximately six months and re-vaccination is required in endemic areas [6,16]. Other preventive measures include implementing movement restrictions on animals and animal products, and culling of infected and contact animals [16]. Biosecurity measures, including active and passive surveillance and disinfecting equipment (e.g., transport vehicles, cattle pens), can also help prevent transmission. |
| Available treatment | There is currently no available treatment, except for supportive care in endemic countries [19,45]. |
| Comparison with past outbreaks | The last reported FMD outbreak in Indonesia occurred in 1983. The outbreak began in cattle, buffalo, and sheep in Cepu, and resulted in 13,976 cases [46,47]. With mass vaccination, movement restrictions, culling and disinfection, Indonesia regained FMD-free status in 1986 [47]. FMDV serotype O also caused the 2001 UK epidemic, which cost USD$20 billion in lost trade, outbreak management costs, and tourism impacts [6,48]. Sheep were the main transmitters during this outbreak and were marketed in large numbers, resulting in widespread transmission before the first case notification [48]. Other contributing factors included [48]:
The current Indonesian outbreak shares some similarities with the 2001 UK epidemic, such as suspected FMD circulation for weeks before initial notification and large movements of animals and people [13]. However, cattle are the main transmitters and vaccination is the main control strategy, alongside culling, disinfection, and movement restrictions of FMD-vulnerable cloven-hoofed animals and animal products[49,50]. |
| Unusual features | This is Indonesia’s first reported FMD outbreak in nearly 40 years. Most livestock in Indonesia are owned by smallholder farms or villages across approximately 6,000 inhabited islands [51]. It is thus difficult to track illegal movements of animals into and within Indonesia. The Indonesian government aims to procure 28.7 million vaccine doses by the end of the year [50], prioritising vaccination for uninfected animals in FMD-affected and surrounding areas [52]. They have also implemented a traffic-light system of zone-based movement restrictions based on vaccination and disease status to prevent FMD spread into unaffected zones [53]. However, it is difficult to track livestock vaccination status, particularly in rural areas. Although ear-tagging identification has begun, owners have been tying red ribbons around necks of cattle to signify vaccination [54]. However, these ribbons may be easily lost. The outbreak also occurred during Eid al-Adha celebrations during April-May and July. Eid al-Adha traditions involve slaughtering of animals and sharing meat with family, friends and community members. Thus, large movements of unvaccinated animals during these celebrations may have majorly contributed to widespread FMD transmission. |
| Critical analysis | The current FMD outbreak is causing significant economic and biosecurity concerns for Indonesia, with forecasted losses of USD$1.37-6.60 billion [13,55]. Without prior routine vaccination [56], Indonesian livestock were completely susceptible to FMD infection [57]. Although Indonesian authorities have identified the current outbreak strain affecting livestock [57], importing or locally producing sufficient doses of an effective vaccine will take time. Additionally, as Indonesia is an archipelago nation [51], ensuring cold chain efficiency during FMD vaccine storage and transport may be difficult [57,58]. Farmers may also be reluctant to vaccinate livestock due to uncertainties regarding vaccine effectiveness, lack of vaccine affordability or insufficient training in administering vaccines [59,60]. Furthermore, since vaccination does not protect against carriage or heterologous infection, the potential for continued outbreak propagation is concerning. Carriage prevalence in cattle is reportedly between 15-50% [17]. Therefore, culling vaccinated animals may also help to safeguard against persistence, simplify post-outbreak surveillance and expedite recovery of FMD-free status [61-63]. Another challenge for containing this outbreak is a shortage of field veterinarians in affected areas, resulting in delayed management of cases [64], which could contribute to further spread of disease. Thus, there is a need for authorities to provide additional training, resources, and if possible, personnel, to affected areas. Field veterinarians and other personnel should also ensure biosecurity and decontamination measures are implemented thoroughly to prevent further indirect FMDV transmission. The illegal importation of animals is suggested to have caused the current FMD outbreak. Illegal wildlife trade is prominent in Indonesia, due to weak policies and inadequate monitoring, enforcement, and information sharing [65,66]. Thus, measures addressing future FMD or other animal disease outbreaks should also consider the driving socioeconomic and governmental factors behind illegal movements of animals [67]. Indonesian livestock farmers and industries will continue to face challenges post-outbreak. Restocking is a lengthy process, involving thorough decontamination, followed by a minimum destocking period, reintroduction of livestock from FMD-free zones (preferably with sentinel animals initially), then intense active surveillance until FMD-free status is regained [68]. The Indonesian outbreak is also causing biosecurity concerns for neighbouring countries, such as Australia, as high tourist traffic could cause FMD-incursion. Australia has already reported detections of FMD viral fragments: in an undeclared beef product carried by a passenger arriving in Adelaide from Indonesia, and in Melbourne pork products illegally imported from China [69,70]. Since Australia has been FMD-free for 150 years, an outbreak could have severe consequences for the livestock industry, with forecasted losses of AUD$80 billion [60,71,72], underscoring the need for stringent surveillance and biosecurity measures. FMD may spread rapidly with serious socioeconomic and trade impacts [73]. Rapid outbreak containment is crucial, requiring disease surveillance and control measures throughout all impacted and at-risk areas, including rural and poor communities. Co-ordinated, multilateral control measures should involve comprehensive consultation and communication with stakeholders in order to be successful. Ongoing monitoring and continued attention to biosecurity measures will be crucial for early detection and management of any further cases, and, in the long-term, help ensure that Indonesia has met all requirements to regain FMD-free status. After regaining FMD-free status, efforts should be focused on preventing future outbreaks, through robust surveillance and biosecurity regulations, and intersectoral collaboration between government, communities, and individuals, to maintain vigilance against further FMD incursions into Indonesia. |
| Key questions |
|